Journal of Basic and Applied Pharmaceutical Science Volume 2 (2024), Article ID: JBAPS-106
https://doi.org/10.33790/jbaps1100106Research Report
Preliminary Screening of Anxiolytic and Anti-depressant Potential of QintroTM a Polyherbal Formulation on Alcohol Withdrawal Syndrome in Experimental Mice
Rushikesh Sonawane, Gaurav Kasar, Dipti Chavan, Manoj Mahajan, Aman Upaganlawar*, Chandrashekhar Upasani
Department of Pharmacology, SNJB’s Shriman Sureshdada Jain College of Pharmacy, Chandwad, Nashik, India.
Corresponding Author Details: Aman Upaganlawar, Professor, Department of Pharmacology, SNJB’s Shriman Sureshdada Jain College of Pharmacy, Neminagar, Chandwad, Nashik, Maharashtra, India.
Received date: 23rd January, 2024
Accepted date: 20th March, 2024
Published date: 22nd March, 2024
Citation: Sonawane, R., Kasar, G., Chavan, D., Mahajan, M., Upaganlawar, A., & Upasani, C., (2024). Preliminary Screening of Anxiolytic and Anti-depressant Potential of QintroTM a Polyherbal Formulation on Alcohol Withdrawal Syndrome in Experimental Mice. J Basic Appl Pharm Sci, 2(1): 106.
Copyright: ©2024, This is an open-access article distributed under the terms of the Creative Commons Attribution License 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Chronic alcohol consumption is a major cause of mortality, morbidity, and economic consequences globally. Prolonged alcohol use and its sudden withdrawal are considered the triggers for anxiogenic effects in rodents and humans. Also, alcohol withdrawal (AW) is involved in depressive disorders. This study investigates the impact of QintroTM on AW mice who were given anxiety and depression
Methods: There were seven groups of mice (n = 6). 10% (v/v) ethanol was administered twice daily at a 2000 mg/kg dose, intragastrically on day 1 and once a day up to day 6. Other groups were given a 10% ethanol treatment (2000 mg/kg p.o.). with test formulation at different doses (mg/kg) designated Q-100, Q-200, Q-400, p.o. They were withdrawn from ethanol on the 7th day, and behavior was analyzed using the tests, which include the tail suspension test (TST), marble burying test (MBT), hole board test (HBT), elevated plus maze (EPM), and light-dark test (LDT).
Results: This investigation showed the beneficial effects of QintroTM on current conditions. The concentration of QintroTM used in the current research revealed significant differences (p<0.05) in time devoted (sec) in the open arm of EPM, time devoted in the bright region in LDT, and head droops in HBT. Additionally, when comparing the TST group to the AW group, there were substantial (p<0.05) decreases in MBT and reductions in immobility time.
In summary: The results of the study showed that QintroTM has both antidepressant and anxiolytic effects in mice who are ethanol deprived.
Keywords: Alcohol withdrawal (AW), Anxiety, Depression, QintroTM, polyherbal formulation, behavioral study.
Introduction
The most prevalent psychiatric disorders worldwide are anxiety and depression, which negatively impact the quality of life [1,2]. Nearly 10% of the world's population is affected by anxiety and depressive disorders annually, which are widespread and debilitating psychiatric diseases [3]. The symptoms of anxiety and depression can coexist, and vice versa [4].
Ethanol is the most common alcohol among chronic consumers who may experience the adverse effects of excessive alcohol use [5]. It is now well known that chronic alcohol intake may lead to behavioral and biochemical alterations in humans and animals. Moreover, regular consumers are prone to developing AW syndrome, which is caused by the cessation of chronic intake. Alcohol withdrawal syndrome (AWS) is characterized mainly by anxiety, along with depression, agitation, hypertension, nausea, vomiting, hallucinations, insomnia, delirium, sweating, tachycardia, and tremors [6,7]. AWS is a commonly occurring state that progresses after severe or regular alcohol consumption is immediately stopped, intentionally or unintentionally [8,9].
Alcohol-induced imbalances in the neurotransmitters cause higher neuronal activity if the alcohol is revoked [10]. In humans, withdrawal significantly lowers brain GABA concentrations [11]. Alcohol increases the effect of GABA on GABAA receptors, which reduces overall brain excitability. Activation of the amygdala and hypothalamus is considered to play a leading role in treating AW anxiety-like behaviors in various limbic regions [6]. The central neurons that make serotonin (5HT) and dopamine (DA) may also experience neuroadaptive alterations brought on by alcohol. When alcohol is consumed, the dopaminergic and serotonergic pathways undergo the opposite adaptive changes as when alcohol is stopped [12].
QintroTM, a polyherbal formulation manufactured by Rudraksha Ayurveda-Pharma, Mumbai, India, is indicated for insomnia, anxiety, and sleep disturbance. The components used in this formulation are popular in Indian and Chinese medicine to treat a variety of neurological disorders. Sarpagandha (Rauwolfia serpentina) is commonly used to treat psychotic illnesses, including anxiety and depression [13,14]. Guduchi (Tinospora cordifolia) has anxiolytic and anti-depressant properties [15,16]. Vacha (Acorus calamus) possesses anxiolytic and antihypertensive properties [17,18]. Bramhi (Bacopa monnieri) has anti-depressant and anti-anxiety activity [19,20]. Tagar (Valeriana wallichii) effectively treats insomnia, anxiety, and depression [21]. Jatamansi (Nardostachys jatamansi) treats mild to moderate sleep difficulties, anxiety, and depressive behavior [22, 23][22,23], and Shankhpushpi (Convolvulus prostratus) has been shown to reduce anxiety associated with AW and alcohol addiction [24,25].
The purpose of this study is to conduct a preliminary screening to evaluate the anxiolytic and antidepressant potential of QintroTM, a polyherbal formulation, in alleviating symptoms of alcohol withdrawal syndrome in experimental mice.
Materials and Methods
Animals
Male mice (albino, 20–25 g) for the study were procured from Lacsmi Biofarms, Pune, India. Mice were housed in polypropylene cages and kept in conditions (room temperature 25±2°C, humidity 45–55%, cycle of light and dark 12:12 hrs). Allow animals to be acclimated to the laboratory for 7 days before starting the experiment. Mice were fed on food pellets, and water was given ad libitum. Food but not water was withheld two hours before the administration of alcohol and the test formulation. All experiments were performed following the CCSEA Laboratory Animal Use and Care Guidelines. The protocol for this investigation was acknowledged by the IAEC (SSDJ/IAEC/2022-23/02).
Drugs and chemicals
QintroTM was purchased from Rudraksha Ayurveda-Pharma, Mumbai, India. The supplier of absolute ethanol was Changshu Hong Sheng Fine Chemical Co. Ltd. in China. The supplier of diazepam was Neon Laboratory Limited, in Thane, India. Imipramine was purchased from Modern Pharmaceutical, Mumbai, India.
Study Design
Six groups of mice were created, with six mice in each group.
Group I: saline (control); Group II: ethanol withdrawal (2000 mg/kg, p.o. 10% v/v) till day 6; and saline on day 7 (ethanol) [26]. Group III: Ethanol (2000 mg/kg, p.o. 10% v/v) till day 6, followed by Q-100 (p.o.) on day 7 (ETH + Q100); Group IV: Ethanol (2000 mg/kg, p.o. 10% v/v) till day 6, followed by Q-200 (p.o.) on day 7 (ETH + Q200); Group V: Ethanol (2000 mg/kg, p.o. 10% v/v) till day 6, followed by Q-400 (p.o.) on day 7 (ETH + Q400); Group VI: Mice treated with ethanol till day 6, followed by diazepam (1 mg/kg, i.p.) or imipramine (10 mg/kg, i.p.) considered as a standard (STD) on day 7 [27].
All the treatments were given from 10.00 to 11.00 a.m. in the morning and for 30 minutes, before carrying out behavioral tests.
Induction of alcohol withdrawal
Mice were given two doses of 10% v/v ethanol, 2000 mg/kg, intragastrically on the first day and once a day up to day 6. Mice were examined for withdrawal symptoms on the seventh day. On the 7th day, the treatment was switched, and the chronic ethanol treatment group received only saline on the 7th day [26].
Assessment of QintroTM for anti-anxiety and anti depression effects
Elevated plus maze: The EPM is an extensively employed behavioral test to examine anxiogenic and anxiolytic behaviors in rodents [28]. The EPM has four arms arranged in the form of '+'. Two arms (open arms: 25×5 cm) don't have side or end walls. The remaining two arms (closed arms: 25×5×15 cm) are open at the top but have side and end walls. At the intersection of the four arms, there is a square platform measuring 5×5 cm. The EPM apparatus was placed 50 cm off the ground [29]. The mice were put in the center, facing an open arm, at the start of each test. The mice were given 5 minutes to explore the EPM to record the duration of stay in each arm and the entries [30].
Light and Dark Test:
The LDT is a widely used rodent test for investigating unconditioned anxiety-like behavior based on a conflict between an accession or evasion urge to explore different regions and an aversion to brilliantly lit, open spaces [31]. There are two compartments on the LDT device. Two-thirds of the box comprises the open, well-lit illumination section. A covered, dark compartment makes up one- third of the entire box. The two compartments are joined by a 7-cm door [32]. The mice were first put in the center of the light [33]. The transitions and the time spent in the darkness to the brightness compartment were captured for a duration of five minutes [34].
Hole board test:
Measurements of a mouse's propensity to poke its nose or snout through holes have been performed using hole boards or hole boxes. One of the noteworthy behaviors is hole-poking, also known as head dipping, which has been proven to be extremely sensitive to drug effects [35]. An HBT apparatus (35 cm x 35 cm x 15 cm) was used to observe the mice's anxiogenic effects. The arena floor is made of black wooden plate with 16 holes divided into 16 equal parts, and the walls are made of wooden sheets (3.5 cm in diameter). The apparatus was lifted 56 cm off the ground. Each mouse was located in the center of the apparatus, and the number of heads dipping was counted for 5 minutes [36].
Marble Burying Test:
The MBT is a widely used experiment examining repeated behavior and a phenotype resembling anxiety [37]. A test for marble burying was performed utilizing a propylene cage and 12 marbles made of clean glass that were spaced evenly, or 4 cm apart, on the husk. QintroTM at varied doses was given to mice 30 minutes before the test. After that, mice were put in the cage with the marbles. After 30 minutes, the number of buried marbles was counted.
Tail Suspension Test (TST):
The TST is a behavioral test for desperation in which mice are positioned 50 cm off the ground by the distal end of their tails. Blinded observers timed their total immobility for six minutes. The immobility time in this test can be decreased by anti-depressants [38].
Statistical Analysis:
The mean and SEM for each group's data is shown. A one-way ANOVA was used for statistical analysis, followed by Dunnette's test utilizing Graph-Pad Prism version 5.0 (USA). Statistics were found to be significant at p values < 0.05. The graphs showed ap< 0.05 in contrast to group I and bp< 0.05 contrasted with group II.
Results
Impact of QintroTM on the exploratory behavior of ethanol withdrawal mice in EPM
As shown in Figure 1, alcohol withdrawal animals exhibited the anxiogenic effects after chronic ethanol consumption, as indicated by significantly (f (5,30)= 40.67, p <0.05) more time invested in the close arm. Contrarily, administration of QintroTM post-withdrawal was observed to reduce anxiety, as evidenced by a considerably (f (5,30) = 50.25, p <0.05) longer open arm time compared to ethanol withdrawal group II.
Effect of QintroTM on the behavior of ethanol withdrawal mice in LDT
Figure 2 illustrates the result of QintroTM on the tested settings in the LDT apparatus. The mice in both the control group (which received saline according to methods) and those experiencing ethanol withdrawal exhibited a significant increase in the amount of time they spent in the dark chamber compared to the light compartment (f (5,30) = 16.78, p< 0.05), suggesting elevated anxiety levels. However, it's important to note that while the withdrawal group experienced anxiety related to alcohol cessation, the control group did not undergo alcohol withdrawal. The anxiolytic efficacy of the polyherbal formulation was indicated by a significant (f (5,30) = 29.18 , p< 0.05) increase in the amount of time mice treated with QintroTM spent in the light chamber. Compared to the vehicle-treated and ethanol withdrawal groups, group V, which got Q-400, exhibited a more notable effect.
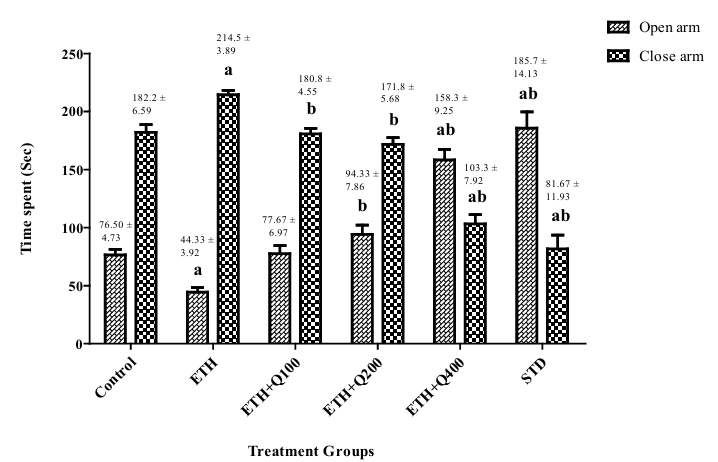
Figure 1. Impact of QintroTM on the behavior of ethanol withdrawal mice in the EPM test. The graph showed a comparison between the open and closed arms, and the bar represented the mean (n = 6) of time spent (sec) in each arm.
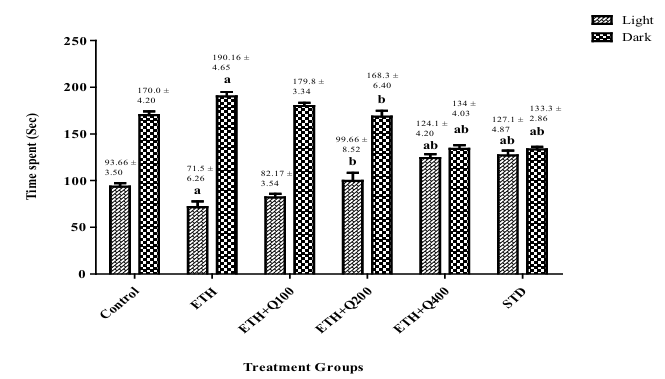
Figure 2. Impact of QintroTM on behavior of ethanol withdrawal mice in LDT. The graph showed a comparison between the light and dark compartments, and the bar represented the mean (n = 6) of time spent (sec) in each compartment.
Effect of QintroTM on the behavior of ethanol withdrawal mice in HBT
Figure 3 illustrates the results on specifications tested in the HBT apparatus. The control and ethanol withdrawal mice exhibited a significant decrease in head dips (f (5,30) = 20.63, p< 0.05), indicative of anxious behavior stemming from abstinence. However, it is noteworthy that while the withdrawal group experienced anxiety related to alcohol cessation, the control group did not undergo alcohol withdrawal. Mice treated with QintroTM revealed a significant (p< 0.05) increase in head dips in mice, demonstrating the polyherbal formulations anxiolytic activity. A stronger impact was observed in group V Q-400 as opposed to vehicle-treated groups and groups that disengage from ethanol.
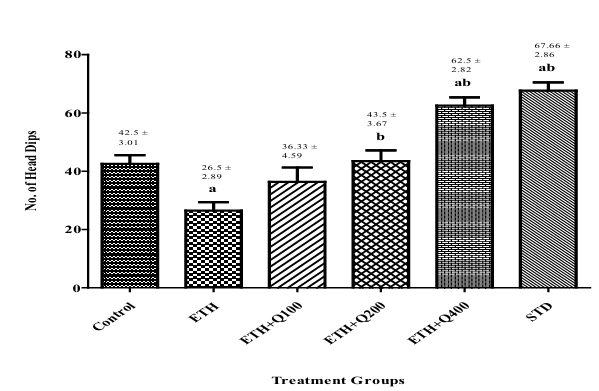
Figure 3. Impact of QintroTM on behavior of ethanol withdrawal mice in HBT. The graph showed the comparison of group I to groups II, III, IV, V, VI and group II to group III, IV, V, VI and the bar represented the mean (n=6) of the numbers of head dips in each group.
Effect of QintroTM on the behavior of ethanol withdrawal mice in MBT
In mice, continuous consumption of ethanol decreased marble burying in MBT in mice compared to group I. Mice administered with QintroTM (Groups III, IV, and V) and groups IV and V showed a significant (f (5,30) = 15.51, p< 0.05) decrease in marble-burying com pared to Group II. Mice treated with Q-400 had a more notable im pact, suggesting that QintroTM has anti-anxiety properties in mice that remove ethanol (Figure 4).
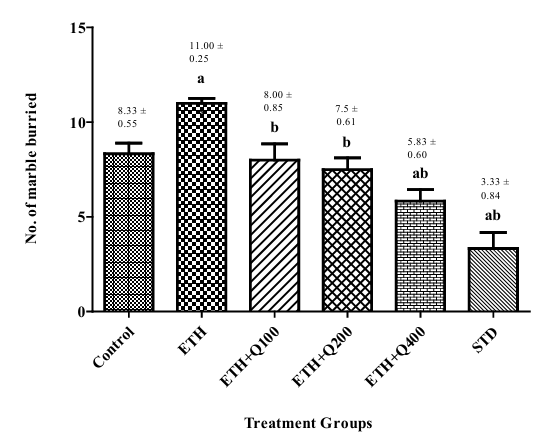
Figure 4. Effect of QintroTM on behavior of ethanol withdrawal mice in MBT. The graph showed the comparison of group I to groups II, III, IV, V, VI and group II to group III, IV, V, VI, and the bar represented the mean (n=6) of the number of marble buried in each group.
Effect of QintroTM on the behavior of ethanol withdrawal mice in TST
As shown in Figure 5, alcohol withdrawal animals exhibited anxiogenic effects after chronic ethanol consumption, as indicated by a significant (f (5,30) = 13.13 , p< 0.05) raised immobility moment in the TST. Contrarily, administration of QintroTM post-withdrawal was observed to reduce immobility, as evidenced by a considerable (p< 0.05) decreased inactivity duration in contrast to group II.
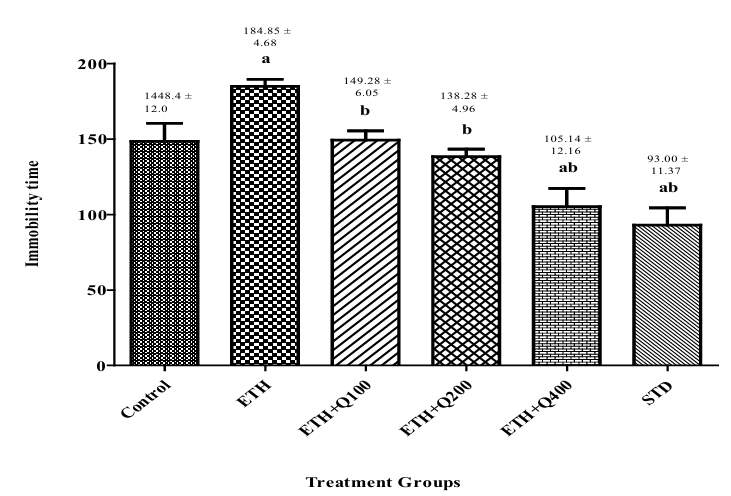
Figure 5. Effect of QintroTM on behavior of ethanol withdrawal mice in TST. The graph showed the comparison of group I to groups II, III, IV, V, and VII and group II to group III, IV, V, and VI and the bar represented the mean (n=6) of immobility time in each group.
Discussion
In the current investigation, we investigated the effect of QintroTM on the anxiety and depression induced by AW in mice. It has been observed that chronic ethanol treatment followed by withdrawal results in anxious-like behavior. AW is induced by an abrupt cessation of drinking after prolonged alcohol consumption. Thus, abstinence develops due to prolonged, excessive ethanol use and withdrawal. Several researchers have shown the link between AW and disruption of ion channel function and changes in neurotransmitter levels in the brain, leading to the development of anxiety and depression [39-41].
AW has been associated with decreased GABAergic transmission and down-regulated GABAA receptors, creating the hyper glutamatergic state, which, when combined with decreased GABA function, results in excessive excitatory signaling and AW anxiety [42]. The characteristic of anti-addictive substances is that they inhibit withdrawal syndrome [43]. We also evaluated whether QintroTM can mitigate anxiety-like behavior associated with AW. According to data from the EPM, LDT, HBT, MBT, and TST, these are well validated models to screen the agents used for relieving anxiety like behavior. In the current investigation, QintroTM significantly reversed the AW-induced anxiety in a dose-dependent manner in all the models [44]. It is a combination of seven components with a variety of pharmacological effects. It's possible that the sum of all these things helped to alleviate withdrawal symptoms.
The anxiolytic activity may be due to its components, including Sarpagandha (Rauwolfia serpentina) [13], Vacha (Acorus calamus) [18], Jatamansi (Nardostachys jatamansi) [23], and Shankhpushpi (Convolvulus prostratus). These components showed an anti-anxiety effect in ethanol withdrawal and alcohol-addicted mice [25]. Most of these herbs have previously been shown to modulate the GABAergic system and provide protection against anxiety and addiction [13,18,23].
In the present work, AW mice was evaluated by TST. AW mice exhibited more immobility time, indicating a depressive effect due to sudden alcohol cessation [45]. On the other hand, QintroTM treatment decreases the duration of immobility, indicating the anti-depressant effect of this polyherbal formulation.
The components of QintroTM, like Guduchi (Tinospora cordifolia) [16], Bramhi (Bacopa monnieri) [20], and Tagar (Valeriana wallichii) [21], were previously shown to possess anti-depressant activity. Most of these herbs modulate the serotonin (5-HT) level and provide protection against depression [46].
Taken together, our study has shown the positive effect of QintroTM in mice where alcohol abstinence caused anxiety and despair and might be useful as an anti-addiction remedy, especially in individuals with alcohol addiction.
Conclusion
Our results suggest that prolonged alcohol intake and sudden withdrawal are associated with the induction of anxiety and depression in laboratory mice. Whereas, administration of this polyherbal formulation may help prevent the development of tolerance to and dependence on ethanol. To sum up, these results provide the initial proof of the anti-addiction property of QintroTM, and it may be helpful against protection from AW syndrome and associated behavioral and biochemical changes.
Funding:
There was no external funding to conduct this study.
Acknowledgement
The authors express gratitude to the Management of SNJB's College of Pharmacy for providing the necessary facilities and Dr. Gaurav Thakor (MD), for providing QintroTM a polyherbal formulation and guidance for the experimental work.
Conflicts of Interest:
According to the writers, there is no conflict of interest.
References
Alsaad, R., Aziz, S., Ahmed, A., Denecke, K., Househ, M., Farooq, F., & Sheikh, J. (2022). Abd-alrazaq A. Wearable Artificial Intelligence for Anxiety and Depression, 25, 42672. View
Biagianti, B., Foti, G., Di Liberto, A., Bressi, C., & Brambilla, P. (2023). CBT-informed psychological interventions for adult patients with anxiety and depression symptoms: A narrative review of digital treatment options. Journal of Affective Disorders, 325, 682–694. View
Simpson, C. A., Diaz-Arteche, C., Eliby, D., Schwartz, O. S., Simmons, J. G., & Cowan, C. S. M. (2021). The gut microbiota in anxiety and depression – A systematic review. Clinical Psychology Review, 83, 101943. View
Liu, X., Zhao, W., Hu, F., Hao, Q., Hou, L., Sun, X. (2022). Comorbid anxiety and depression, depression, and anxiety in comparison in multi-ethnic community of west China: Prevalence, metabolic profile, and related factors. Journal of Affective Disorders, 298(A), 381–387. View
Xiao, H. W., Ge, C., Feng, G. X., Li, Y., Luo, D., Dong, J. L., . . . Fan, S. J. (2018). Gut microbiota modulates alcohol withdrawal-induced anxiety in mice. Toxicology Letters, 287, 23–30. View
Sharma, L., Sharma, A., Gupta, G., & Bisht, G. (2018). Protective effect of ocimum sanctum linn. Leaf Extract on ethanol withdrawal syndrome in Wistar rats. Asian Pacific Journal of Tropical Medicine, 11(8), 467–472. View
Meza, V., Arnold, J., Díaz, L. A., Ayala Valverde, M., Idalsoaga, F., Ayares, G., . . . Arab, J. P. (2022). Alcohol consumption: Medical implications, the liver and beyond. Alcohol and Alcoholism, 57(3), 283–291. View
Jesse. A, Brathen. G, Ferrara. M, Keindl. M, Ben-Menachem, Tanasescu. R, Brodtkorb, Hillbom. M, Leon. M. A, Ludolph. A. C, (2016). Alcohol withdrawal syndrome: mechanisms, manifestations, and management. Acta Neurologica Scandinavica. 135(1):4-16. View
Gupta, G. L., & Rana, A. C. (2008). Effect of Withania somnifera Dunal in ethanol-induced anxiolysis and withdrawal anxiety in rats. Indian Journal of Experimental Biology, 46(6), 470–475. View
Pati, D., Marcinkiewcz, C. A., DiBerto, J. F., Cogan, E. S., McElligott, Z. A., & Kash, T. L. (2020). Chronic intermittent ethanol exposure dysregulates a GABAergic microcircuit in the bed nucleus of the stria terminalis. Neuropharmacology, 168, 107759. View
McGinnis, M. M., Parrish, B. C., & McCool, B. A. (2020). Withdrawal from chronic ethanol exposure increases postsynaptic glutamate function of insular cortex projections to the rat basolateral amygdale. Neuropharmacology, 172, 108129. View
Mierzejewski, P., Bienkowski, P., Jakubczyk, A., Samochowiec, J., & Silczuk, A. (2022). Pharmacotherapy of alcohol withdrawal syndromes – Recommendations of the Polish Psychiatric Association and the Pharmacotherapy Section of the Polish Society for Addiction Research. Pharmacotherapy of alcohol withdrawal syndromes, 276, 1–20. View
Ali, W. B., Shireen, E., Masroor, M., Kiran, S., Memon, N., Junaid, N., & Haleem, D. J. (2022). Oral administration of Rauwolfia serpentina Plant extract mitigated immobilisation stress-induced behavioral and biochemic and deficits in rats p. 12(1):32. Biology and Life Science Forum. doi:10.3390/IECN2022-12393 View
Lakshmipriya, M., Kokilambigai, S., & Ilango, K. (2023). A systematic review of analytical methods for quantification of natural indole alkaloids from Catharanthus and Rauvolfia species. Research Journal of Pharmacognosy, 10(1), 57–66. View
Patil, S., Trigunayat, A., & Chaudhary, A. (2015). Evaluation of anxiolytic and antidepressant effect of different dosage forms of the Guduchi. International Journal of Green Pharmacy, 9(4), 25–30.View
Devade, O., & Londhe, R. (2022). Medicinal plants with Memory enhancing activity [Review]. Journal of Pharmaceutical Advance Research, 5(2), 1452–1459.
Jain, D., Gupta, S., Jain, R., & Jain, L. (2010). Anti-inflammatory Activity of 80% ethanolic Extract of Acorus calamus Linn. Leaves in albino rats. Research Journal of Pharmacy and Technology, 3, 882–884. View
Sharma, V., Sharma, R., Gautam, D. S., Kuca, K., Nepovimova, E., & Martins, N. (2020). Role of Vacha (Acorus calamus linn.) in neurological and metabolic disorders: Evidence from ethnopharmacology, phytochemistry, pharmacology and clinical study. Journal of Clinical Medicine, 9(4), 1176. View
Ragini. S., (2023). Herbal medicines and anxiety disorders. International Journal for Multidisciplinary Research, 5(1), 1–10. View
Ahmad, S. R., & Karmakar, S. (2023). The role of medicinal plants in drug discovery across the world. Indian Journal of Pure and Applied Biosciences, 11(2), 30–41. doi:10.18782/2582 2845.8995 View
Singh, V., Singh, D., Tiwari, R., & Vashishtha, K. (2023). A conceptual review on Tagar (Valeriana wallichii DC.) and it’s medicinal properties. World Journal of Pharmaceutical Research, 12(2), 349–358. View
Jha, S. (2020). Identification of chemical composition of plant extracts of Nardostachys Jatamansi DC using GC/MS. IOSR Journal of Pharmacy and Biological Sciences, 15(5), 32–35. View
Wang, S. N., Yao, Z. W., Zhao, C. B., Ding, Y. S., Jing-Luo, J., Bian, L. H. (2021). Discovery and proteomics analysis of effective compounds in Valeriana jatamansi jones for the treatment of anxiety. Journal of Ethnopharmacology, 265, 113452. View
Heba, M., Faraz, S., & Banerjee, S. (2017). Effect of Shankhpushpi on alcohol addiction in mice. Pharmacognosy Magazine, 13(1) Suppl. 1, S148–S153. View
Agarwa, P., Sharma, B., Fatima, A., & Jain, S. K. (2014). An update on Ayurvedic herb Convolvulus pluricaulis Choisy. Asian Pacific Journal of Tropical Biomedicine, 4(3), 245–252. View
Joshi, D., Naidu, P. S., Singh, A., & Kulkarni, S. K. (2005). Protective effect of quercentin on alcohol Abstinence-Induced Anxiety and depression. Journal of Medicinal Food, 8(3), 392 396. View
Doukkali, Z., Taghzouti, K., Bouidida, E. L., Nadjmouddine, M., Cherrah, Y., & Alaoui, K. (2015). Evaluation of anxiolytic activity of methanolic extract of Urtica urens in a mice model. Behavioral and Brain Functions, 11, 19. View
Fabris, D., Carvalho, M. C., Brandão, M. L., Prado, W. A., Zuardi, A. W., Crippa, J. A., . . . Genaro, K. (2022). Sex dependent differences in the anxiolytic-like effect of cannabidiol in the elevated plus-maze. Journal of Psychopharmacology, 36(12), 1371–1383. View
Fathalizade, F., Baghani, M., Khakpai, F., Fazli-Tabaei, S., & Zarrindast, M. R. (2022). GABA-ergic agents modulated the effects of histamine on the behaviour of male mice in the elevated plus maze test. Experimental Physiology, 107(3), 233 242. View
Knight, P., Chellian, R., Wilson, R., Behnood-Rod, A., Panunzio, S., & Bruijnzeel, A. W. (2021). Sex differences in the elevated plus-maze test and large open field test in adult Wistar rats. Pharmacology, Biochemistry, and Behavior, 204, 173168. View
Arrant, A. E., Schramm-Sapyta, N. L., & Kuhn, C. M. (2013). Use of the light/dark test for anxiety in adult and adolescent male rats. Behavioural Brain Research, 256, 119–127. View
Bourin, M., & Hascoët, M. (2003). The mouse light/dark box test. European Journal of Pharmacology, 463(1–3), 55–65. View
Zhou, C., Assareh, N., & Arnold, J. C. (2022). The cannabis constituent cannabigerol does not disrupt fear memory processes or stress-induced anxiety in mice. Cannabis and Cannabinoid Research, 7(3), 294–303. View
Doukkali, Z., Taghzouti, K., Kamal, R., Jemeli, M., Bouidida, E., Zellou, A., . . . Alaoui, K. (2016). AntiAnxiety Effects of Mercurialis annua Aqueous Extract in the Elevated plus maze test. Journal of Pharmacological Reports, 1, 1–5. View
Chapter, K. A. (1993);10. Locomotor activity and exploration. Techniques in the Behavioral and Neural Sciences, 19:499-518. View
Ibironke, G., & Olley, S. (2014). Cholinergic modulation of restraint stress induced neurobehavioral alterations in mice. African Journal of Biomedical Research, 17, 181–185. View
Wahl, L., Punt, A. M., Arbab, T., Willuhn, I., Elgersma, Y., & Badura, A. (2022). A novel automated approach for improving standardization of the marble burying test enables quantification of burying bouts and activity characteristics. eNeuro, 9(2), 0446–0421. View
Ramos-Hryb, A. B., Pazini, F. L., Costa, A. P., Cunha, M. P., Kaster, M. P., & Rodrigues, A. L. S. (2022). Role of heme oxygenase-1 in the antidepressant-like effect of ursolic acid in the tail suspension test. Journal of Pharmacy and Pharmacology, 74(1), 13–21. View
de Brouwer, G., Fick, A., Harvey, B. H., & Wolmarans, W. (2019). A critical inquiry into marble-burying as a preclinical screening paradigm of relevance for anxiety and obsessive–compulsive disorder: Mapping the way forward. Cognitive, Affective and Behavioral Neuroscience, 19(1), 1–39. View
Perez, E. E., & De Biasi, M. (2015). Assessment of affective and somatic signs of ethanol withdrawal in C57BL/6J mice using a short-term ethanol treatment. Alcohol, 49(3), 237–243. View
Mohan, L., Rao, U. S., Gopalakrishna, H. N., & Nair, V. (2011). Evaluation of the anxiolytic Activity of NR-ANX-C (a Polyherbal Formulation) in ethanol Withdrawal-InducedAnxiety Behavior in Rats. Evidence-Based Complementary and Alternative Medicine: eCAM, 2011, 1–7. View
Sharma, L., Sharma, A., Dash, A. K., Bisht, G. S., & Gupta, G. L. (2021). A standardized polyherbal preparation POL-6 diminishes alcohol withdrawal anxiety by regulating Gabra1, Gabra2, Gabra3, Gabra4, Gabra5 gene expression of GABAA receptor signaling pathway in rats gene expression of GABAA receptor signaling pathway in rats. BMC Complement Medicine and Therapies, 21(1), 13. View
Bansal, P., & Banerjee, S. (2016). Effect of Withinia somnifera and shilajit on alcohol addiction in mice. Pharmacognosy Magazine, 12(2) Suppl. 2, S121–S128. View
Gupta, G. L., & Sharma, L. (2019). Bacopa monnieri abrogates alcohol abstinence-induced anxiety-like behavior by regulating biochemical and Gabral, Gabra4, Gabra5 gene expression of GABAA receptor signalling pathway in rats. Biomedicine and Pharmacotherapy, 111, 1417–1428. View
Shetty, R. A., & Sadananda, M. (2022). Effect of Mentone® on depression- and anxiety-like profiles and regional brain neurochemistry in the adolescent Wistar Kyoto rat, a putative model of endogenous depression. Indian Journal of Physiology and Pharmacology, 66(2), 103–110. View
Kumar, R., Ilango, K., Singh, G. P. I., & Dubey, G. P. (2019). Pharmacological in vivo test to evaluate the antidepressant activity of polyherbal formulation. Nepal Journal of Biotechnology, 7(1), 63–73. View