Journal of Basic and Applied Pharmaceutical Science Volume 2 (2024), Article ID: JBAPS-108
https://doi.org/10.33790/jbaps1100108Review Article
Modern Technological Approaches and Applied Complexes of Excipients in The Production of Orodispersible Tablets
Gorbunova Yulia Vasilievna1, Sepp Vladislav Valentinovich2*, Bakulin Konstantin Sergeevich2, Ubushaev Sanji Viktorovich2, and Zharchenko Zarina Sergeevna2
1Federal State Budgetary Educational Institution of Higher Education "Russian University of Medicine" of the Ministry of Health of the Russian Federation (FSBEI HE "ROSUNIMED" OF MOH OF RUSSIA)
2Federal State Autonomous Educational Institution of Higher Education «N.I. Pirogov Russian National Research Medical University» of the Ministry of Health of the Russian Federation (Pirogov Russian National Research Medical University)
Corresponding Author Details: Sepp Vladislav Valentinovich, Associate Professor, Department of Pharmacy, Russian National Research Medical University, Russia.
Received date: 15th July, 2024
Accepted date: 11th September, 2024
Published date: 13th September, 2024
Citation: Vasilievna, G. Y., Valentinovich, S. V., Sergeevich, B. K., Viktorovich, U. S., & Sergeevna, Z. Z., (2024). Modern Technological Approaches and Applied Complexes of Excipients in The Production of Orodispersible Tablets. J Basic Appl Pharm Sci, 2(1): 108.
Copyright: ©2024, This is an open-access article distributed under the terms of the Creative Commons Attribution License 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Modern approaches to the treatment of patients based on a high degree of individualization of therapy, attention to patient compliance and ease of use of drugs determine the vector for the development and further improvement of traditional dosage forms, such as tablets. Optimization of the composition and technological processes allows the creation of highly effective, stable and easy-to use tablets dispersed in the oral cavity (orodispersible tablets, ODT). To obtain them, both traditional tablet manufacturing technologies, such as direct compression, compression with preliminary wet or structural granulation, and some innovative technological processes are applicable. The composition of excipients in ODT is maximally unified with conventional tablets, differing mainly in the quantitative ratios of the components, in particular, a significant increase in the proportion of disintegrants and the use of superdisintegrants. A reasonable expansion of the range of tablet dosage forms due to ODT is a rather promising direction in the development of tablet technology.
Keywords: Orodispersible Tablets, Tableting, Excipients, Tableting Methods, Direct Compression
Relevance of ODT development and scope of application
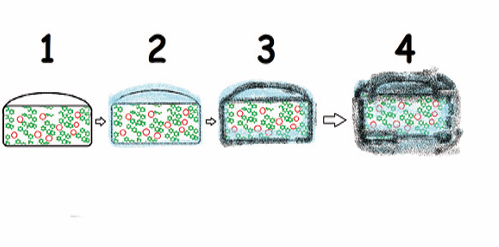
The modern pharmaceutical industry continues to actively work on the development and improvement of tablet dosage form technology. Currently, various innovative technologies such as extrusion, coagulation, electrostatic coating and others are actively used to create new forms and variations of tablets. One of the important trends in the development of tablet technology is the creation of tablets with controlled release of the drug substance. Such tablets provide prolonged action and reduce the number of doses per day. In addition, there are new forms of tablets with different flavors and aromas, which increases the convenience of taking medicines, especially for children. The decomposition process of ODTs in a limited amount of liquid is shown in Figures 1 and 2.

Figure 2. Photographs of the disintegration of ODTs in small amounts of water without water agitation (taken from Kantharao, C. H., Swarna, K., Leelakrishna, J., Anusha, J., Asha, B., & Bhavani, B. (2019). Diclofenac orodispersible tablets: Formulation and in vitro evaluation. Ann Clin Lab Res, 7, 1-8.).
The development of tablet technology is also inextricably linked to the use of new excipients. For example, the use of nanoparticles as excipients can improve the bioavailability and reduce the dosage of drugs [1,2].
Thus, the development of tablet dosage form technology is an important direction in the pharmaceutical industry and continues to attract great interest from specialists. One of the most successful modifications of tablet dosage forms, which combines simplicity and manufacturability of production with improved patient comfort, is orodispersible tablets (ODT). Absorbable tablets (ODTs) and absorbable films (ODFs) are solid oral dosage forms that rapidly disintegrate or dissolve when placed in the mouth [3]. They are designed to make it quick and easy to take the medicine, especially for patients who have difficulty swallowing. In addition, ODTs may have a pleasant taste and flavor, which enhances ease of administration, especially in children. The development of ODT production technology continues to attract a great deal of interest from scientists and pharmaceutical industry professionals. They are looking for new ways to produce ODTs that will improve their quality, stability, bioavailability and safety, as well as increase productivity and reduce the cost of production. Some of the innovative technologies for the production of ODTs include extrusion technology, which can be used to create ODTs of different shapes and sizes, and the use of nanoparticles, polymers, solvents and other excipients that improve the bioavailability, solubility and stability of the drug substance [4-7].
This dosage form provides faster delivery of the active ingredient due to rapid disintegration of the dosage form at the beginning of the GI tract and rapid passage of the gastric gatekeeper. The risk of choking or spasm during oral administration of traditional drugs due to physical blockade disappears, which contributes to the safety of orodispersible drug administration. An additional advantage of these tablets is that they do not need to be soaked with water when taken [8,9]. Due to rapid disintegration in the initial parts of the gastrointestinal tract, the onset of therapeutic effect after ODT administration compared to traditional dosage forms can be more predictable and faster [10,11]. This dosage form allows to avoid social stigmatization in cases where there are objective prerequisites for this (for example, in the presence of mental pathology). In this regard, the results of one of the studies analyzing the preferences and needs of patients in the course of treatment are quite indicative: 70% of patients ask their physician to prescribe ODTs, 70% purchase this form of medication from pharmacies, and more than 80% prefer ODTs when given a choice between ODTs and conventional tablets and solutions [11]. Thus, the use of orally dispersible dosage forms avoids some of the factors that reduce adherence to therapy (hurry, lack of water for drinking, presence of strangers).
Dosage forms suitable for children are indispensable in modern medicine and are a prerequisite for successful paediatric drug therapy. For many years, experts have called for a paradigm shift, from liquid dosage forms to new oral solid dosage forms. ODTs have significant potential as dosage forms in paediatric therapy that has not yet been fully unlocked [13].
However, not all active substances can be used in the form of orodispersible tablets, due to the specific features of this form. When interacting with saliva, drugs undergo enzymatic, acidic or alkaline action, and the digestion process begins. This affects the bioavailability of the drug, which can vary depending on the productivity of salivary gland secretion, salivary pH and age-related changes in oral tissues. In addition, not all active substances are able to be fully absorbed in the oral cavity, part of the dose enters the lower GI tract through reflexive swallowing movements. Some active substances may limit their absorption due to factors such as dose, molecular weight, solubility in water and saliva, degree of ionization at pH 6.8, ability to diffuse through the epithelium and ability to dissolve in blood [9].
Additional requirements for ODTs (in addition to the general and specific requirements for tableted FPs set out in the regulatory documentation of FPs) can be formulated as follows. ODTs must:
- disintegrate or dissolve in the mouth in no more than 3 minutes without drinking water;
- be pleasant to taste, conceal the taste of the active substance;
- be compact and sufficiently strong (withstand the strength test according to the OFS);
- leave no or minimal undissolved particles in the mouth after use;
- be stable with respect to environmental changes (temperature, humidity);
- be economically attractive to the manufacturer.
Physically, a tablet that is dispersed in the oral cavity is a porous body. When immersed in liquid (saliva), it penetrates every capillary with which the tablet is impregnated and causes it to break down very rapidly [12].
Technologies for obtaining ODT
The main ways to achieve the effects of rapid dissolution and rapid disintegration, and, consequently, to increase the bioavailability of orodisperse drugs are currently:
- the use of special excipients such as gas-forming mixtures, complexones, solubilizers or superdisintegrants;
- use of special technological methods such as sublimation, cryo milling, ultrasonic micronization and some others [14].
Lyophilization technology creates a tablet with a readily soluble amorphous porous structure. This is achieved by evaporating the water from the polymer matrix, thus producing a porous structure. The active ingredient is added to an aqueous solution of the carrier polymer and dispersed or dissolved therein. The resulting mixture is then dispensed into the pre-manufactured cells of the blister pack. The blister packs containing the drug solution are frozen with liquid nitrogen and placed on trays or pallets. Then they are placed in freezing chambers for further lyophilic drying under deep vacuum conditions. After that blister packs are sealed with aluminum foil using special packaging equipment. The resulting oral lyophilizates have instant disintegration, good absorption and high bioavailability. The lyophilization method has significant disadvantages including high cost and duration of the manufacturing process. Due to the fragility of the drug, traditional blister packaging cannot be used; special packaging is required in which the cells are opened using a foil segment. In addition, the drugs are unstable to changes in temperature and humidity [15,16].
An example of a lyophilized ODT is the Zydis® technology, which uses natural polymers such as gelatin, dextran or alginates to provide strength and elasticity. In addition, saccharides such as mannitol or sorbitol are used to give structure to the tablet. Water is also used in the manufacturing process to create a porous structure in order to achieve rapid disintegration. Various gums are also used to stabilize the drug suspension prior to lyophilization. Cryoprotectants such as glycine prevent Zydis technology tablets from changing shape during lyophilization drying and storage. The patented Zydis® technology produces Motilium in the form of mint-flavored chewable tablets, [17] which dissolve on the tongue in 2-3 seconds.
Preparation of ODTs by molding can take place either using azeotropic method (dissolution method) or heating method [18]. Dissolution method is used to create tablets from powdered drug. In this method, the drug is wetted with an aqueous-alcoholic solution of polymer and then the wet mass is compressed into special molds under low pressure. The water-alcohol solvent is then removed by drying or evaporation. These tablets are classified as trituration tablets according to the Russian classification. Tablets produced by this method are less compact, but have a porous structure, which ensures their rapid dissolution.
The heating method consists of preparing a suspension containing the drug, agar-agar and sugar (e.g., mannitol or lactose), dispensing the suspension into blister cells, solidifying the agar-agar at room temperature to form a jelly mass and drying it at 30°C under vacuum. The addition of binders is necessary to increase the mechanical strength of the molded tablets, which is relatively low. In addition, it is desirable to improve the flavor of these tablets. In any case, compared to the lyophilization method, the tablet molding method is cheaper and more productive [12].
Spray drying technology typically uses gelatin as an excipient and matrix, mannitol or lactose as a filler, sodium starch glycolate, croscarmellose or crospovidone as superdisintegrants. The gassing mixture is, for example, a mixture of citric acid and sodium hydrogen carbonate. The powder mixture is spray dried and pressed into tablets. Tablets made by spray drying method dissolve in aqueous medium within no more than 20 seconds [18].
When using sublimation method to create porous matrix, volatile ingredients such as ammonium hydrogen carbonate, ammonium carbonate, benzoic acid, camphor, naphthalene, urea, urethane and phthalic anhydride are first mixed with non-volatile excipients and drug substance, then pressed into tablet form and subjected to sublimation process. Tablets created by this method disintegrate in saliva within 10-20 seconds according to clinical trial results. To improve the mechanical strength of such tablets and to improve their flavor, the addition of binders may be required. However, compared to the lyophilization method, the sublimation method is more cost effective and productive.
Extrusion technology is the extrusion of a softened dispersion of drug in polyethylene glycol through an extruder and dividing the dried cylinder into equal segments or tablets using a heated blade.
Also noteworthy is the technology of ODT roolucation by the Possible Approach method, implemented, in particular, in the work of Tranová T, et al. [19]. Another example of another variant of implementation of freeze drying combined with granulation as a method of obtaining ODTs is the production of ketoprofen tablets [20].
The experience of creating ODTs with granular mannitol in the composition is also of interest. This technological technique allowed to optimise key parameters: Low friability combined with rapid disintegration [21].
Due to the possibility of designing different spatial structures, 3D printing can be implemented in the production of personalised medicines, including ODTs, which was shown on the example of creating ODT with fluconazole [22].
Direct pressing is a simple and economical method for the production of orodispersible tablets, so it is widely used in the production of ODTs. This method is possible due to the availability of high quality excipient ingredients including superdisintegrants and sugar-based excipients, which ensure that the tablets disintegrate instantly in the oral cavity immediately after being placed on the tongue [15,23].
There are patented technologies for the production of ODTs by direct pressing. OraSolve technology is patented by the American pharmaceutical corporation CIMA Labs Inc. Tablets manufactured by this technology contain substances masking unpleasant taste of active substances, fillers, as well as a small amount of gas-forming components (mixtures of sodium hydrogen carbonate and citric acid) necessary to accelerate the process of tablet disintegration in the oral cavity and additional correction of taste. OraSolve technology ODTs are produced by direct pressing at relatively low pressing pressure, which also accelerates the disintegration process. The disadvantage of the technology is the low strength of the tablets [12,40].
DuraSolv technology is a technology also registered by CIMA Labs Inc. Tablets manufactured using this technology consist of an active ingredient, a filler and a lubricant. A gas-forming composition may also be used as a disintegrant, as in OraSolve technology. Tablets are manufactured on conventional pharmaceutical equipment, have high strength (up to 100 N), so they can be packaged in conventional blisters or vials [15].
Flash Dose technology is patented by Fuisz Technologies, Ltd. - an American privately held medical device manufacturing and marketing company. Nurofen mellitic acid salt, a new form of ibuprofen in the form of ODT, manufactured using this technology is the first commercial product launched by Biovail Corp. a Canadian pharmaceutical corporation. Tablets manufactured using Flash Dose technology consist of a binding matrix made from sugar fibers that are mixed with filler and drug substance. The structure is formed when the tablet is heated instantaneously, such as during pressing [24].
WOW Tab technology is patented by the Japanese pharmaceutical company Yamanouchi Pharma Technologies, Inc. The abbreviation WOW stands for With Out Water - without water. This technology utilizes a combination of low and high compressibility and binding capacity saccharides to produce a fast dissolving, strong tablet. The active ingredient is mixed with a readily soluble saccharide (e.g., lactose, glucose, mannitol) and granulated with a saccharide with high binding capacity (e.g., maltose, oligosaccharide) and then pressed into a tablet [18].
FlashTab technology is patented by the French pharmaceutical company Prographarm Group. ODTs produced by this method contain the active substance in the form of microcrystals or microgranules of the drug, which can be manufactured using traditional technologies such as coacervation, microencapsulation, and extrusion. All manufacturing processes used are similar to traditional tablet manufacturing methods.
Auxiliary substances in ODT
In the production of ODTs, mostly the same excipients are used as in the production of traditional tablets. In some cases, the quantitative ratios of the components of the tablet mixture are changed, or additional ingredients are included in the composition [25,39].
Dry binding agents, such as MCC, pregelatinized starch, macrogol 6000, Plasdon S-630, when introduced into the composition of masses provide tabletting of some drug substances without moistening by direct pressing or with the use of dry granulation of tablet mass. MCC is obtained by partial hydrolysis of cotton cellulose with hydrochloric acid. The existing grades of MCC differ in the degree of polymerization; the most commonly used grades are Avicel and Vivapur with a particle size of 50-160 μm [26].
The main peculiarity of the profile of excipients in ODT is the inclusion of a significant amount of loosening agents that contribute to the rapid disintegration of the tablet in the oral cavity, or the use of so-called superdisintegrants, such as structured carboxymethyl cellulose (croscarmellose), sodium starch glycolate (primogel, explotab), polyvinylpyrrolidone (polyplasdone), etc. Significant acceleration of tablet disintegration and dissolution is also promoted by the presence of solubilizers and gas-forming mixtures in the dosage form. According to the mechanism of action, loosening agents are divided into the following groups: swelling agents - agents that rupture the tablet after swelling in contact with liquid; gas forming agents - providing tablet disintegration in liquid medium as a result of carbon dioxide release during the reaction of interaction of components of the gas-forming mixture of substances; improving wettability and water permeability of the tablet and contributing to its disintegration and dissolution [27,45].
Several studies have investigated how the composition and mode of incorporation of superdisintegrants affect wet granulation tablets. The most common superdisintegrants, such as sodium starch glycolate, crospovidone, and croscarmellose sodium, and different modes of incorporation, including extragranular, intragranular, and uniform distribution between phases, were considered. The main component of the tablets were lactose, naproxen or bivalent calcium phosphate to achieve different degrees of solubility of the formulations. The granulates were dried to three moisture levels. The results showed that extragranular incorporation of superdisintegrants resulted in faster dissolution than intragranular incorporation or uniform distribution. The moisture content of the granulate has a specific effect on tablet dissolution depending on the formulation. Each major component of the tablet behaves differently. Sodium croscarmellose tended to dissolve tablets faster than sodium starch glycolate or crospovidone. Superdisintegrants tend to promote faster dissolution in neutral pH medium than in acidic medium when all other factors remained constant [28,29].
Another study analyzed the properties of mouth-dispersible cetirizine tablets produced. Tablets were prepared using cetirizine together with camphor and mannitol in proportions of 1:1:1:1, 1:1:3 and 1:1:6. It was found that the optimum properties of the tablet mixture and the manufactured tablets such as: granule flowability, tablet strength and disintegrability were observed for the formulation with component ratio (1:1:1:3) [30].
The direct pressing method for both traditional tablets and ODTs remains the simplest and therefore the most efficient in terms of product risks and economic costs. If eliminating the granulation stage or carrying out dry granulation does not reduce the quality of the manufactured product and does not carry additional product risks, the direct pressing method is preferred [31].
The use of various saccharide excipients such as dextrose, fructose, isomalt, lactitol, maltitol, maltose, mannitol, sorbitol, starch hydrolysate, polydextrose and xylitol can achieve the desired technological properties of ODTs and adjust the flavor of the drug product. Among saccharides, there are types (e.g., lactose and mannitol) that have low moldability and binding properties but high solubility and types (e.g., maltose and maltitol) that have high moldability and binding properties but low solubility [32].
The most universal composition of excipients is the following: microcrystalline cellulose, lactose monohydrate (milk sugar), glucose, dextrose, aerosil, magnesium stearate or stearic acid, starch. The use of this set of auxiliary substances as a kind of matrix allows the development of compositions of tablet forms of drugs of various names, which are obtained under production conditions by direct pressing [15,32].
Starch is an important excipient in the pharmaceutical industry. It is used as a binding agent during granulation in the form of starch paste solutions with a concentration of 3-10%. In dry form, starch is used as a leavening and antifriction agent. Its loosening action is to increase the porosity of tablets, which promotes the penetration of liquid into them. The round starch grains create a large microporosity in the tablets and its high hydrophilicity provides better penetration of liquid inside the tablets. Starch is usually added to tablets in an amount of up to 30% of the total mass [33,34].
The most widely used excipient for direct pressing is lactose [35]. Different grades of lactose are used in pharmaceutical formulations: spray-dried or crystalline anhydrous beta-lactose and alpha-lactose monohydrate, which have different physical properties and affect the main technological characteristics (friability, compressibility) of tabletting mixtures. When using spray-dried lactose, significantly stronger tablets are obtained than when crystalline varieties are used [36].
Glucose monohydrate, dextrose is a natural organic compound and belongs to the class of monosaccharide carbohydrates. Most often glucose is found in nature in bound form, being a component of high- molecular polysaccharides with the general formula C6H10O5, from which it is obtained on an industrial scale. Glucose (dextrose) food monohydrate is a crystalline hydrated glucose. Glucose monohydrate is a white crystalline powder, sweet to the taste, without extraneous flavor, well soluble in water. It has monoclinic crystals.
In pharmacy it is used as a filler, characterized by good compressibility but poor bulkiness. Due to the shape of its crystals, dextrose tablets have excellent hardness but also increased brittleness [37].
Microcrystalline cellulose (MCC) is a powdery non-fibrous modification of natural cellulose. MCC is obtained by hydrolysis of cellulose with alkalis, acids, acidic salts, and alkali acid treatment. Hydrolysis leads to the destruction of chemical bonds in the amorphous regions of cellulose fibers. Under mechanical action, cellulose fibers are also destroyed in crystalline regions [36,38].
Physicochemical and technological properties of MCC determine its purpose in tablet - to increase the friability of tablet mass, strength of tablets and improve their disintegration [33-35], as well as the method of tablet production - wet or dry granulation, direct pressing or extrusion [43]. Most commonly, MCC is used to increase the compressibility of tablet mixtures in various tablet production methods [43,44]. In this case, the strength of tablets increases with increasing amount of MCC. Cellulose is characterized by a high degree of hydrophilicity and the tendency to form numerous hydrogen bonds between polymer filaments. At the same time, microcrystalline cellulose is more homogeneous, which provides an increased sorption capacity compared to conventional fibrous cellulose [41,42].
Polyvinylpyrrolidone or povidone (PVP) is a water-soluble polymer composed of monomeric units of N-vinylpyrrolidone. PVP is produced through a polymerization reaction from N-vinylpyrrolidone. The chain length of the polymer determines the properties of covidones and their applications. Soluble grades of polyvinylpyrrolidone are considered one of the most versatile excipients in the pharmaceutical industry [44].
Polyvinylpyrrolidone is well soluble in water and is able to adsorb a large number of water molecules. In addition, PVP is soluble in various organic solvents such as alcohols, acetone, cyclohexane, triethylamine, dimethylformamide and dioxane, and here we are talking about the solubility of the dry polymer. However, Polyvinylpyrrolidone containing moisture is not soluble in solvents that are not miscible with water due to hydrate formation. Povidones are insoluble in esters and aliphatic and cyclic hydrocarbons. In addition, long-chain povidones are insoluble in water but can swell. [44].
Polyvinylpyrrolidone is mainly used as a binder for tablets. It is also used as a solubilizing agent to prevent lumping and clumping, as a dispersing agent, and as a stabilizer for temperature-sensitive active ingredients.
Colloidal silicon dioxide (SiO2), a very light micronized powder with pronounced adsorption properties. "Aerosil" is the trade name of the original developer, the German chemical company Evonik Degussa AG. The particle size is 4-40 μm, a very light white powder with a density of 2.2 g/cm3, with a high specific surface area of 50 to 400 m2/g [23,45].
"Aerosil 200" has a specific surface area of 200 m2/g, average particle size of 12 μm, porous and hydrophilic. It is used in tablet mixtures as a sliding and loosening agent. Its addition increases the friability of powders, disintegration, but has a bad effect on the strength of tablets [23,39].
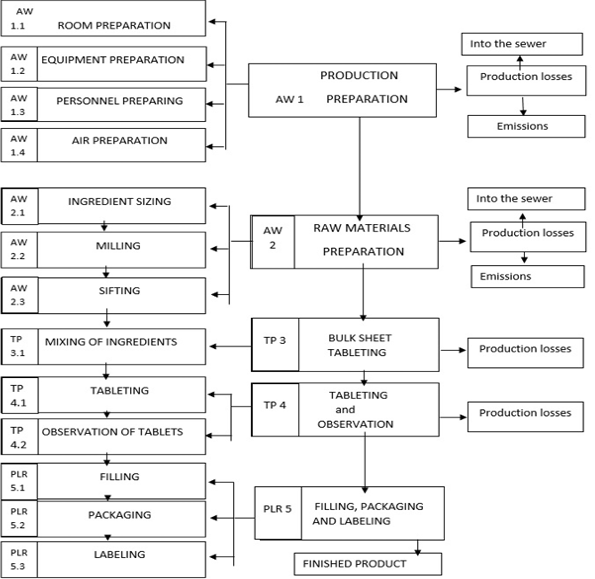
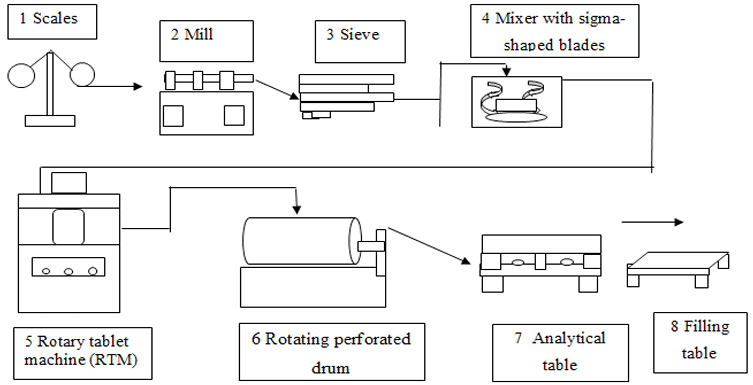
Principal technological and apparatus schemes for the production of ODT by direct pressing are presented in Figures 1-2.
Conclusions
Thus, summarising the results of the above studies, it can be concluded that oral dispersible tablets are becoming increasingly popular among pharmacologists and pharmaceutical professionals due to their unique properties. They dissolve in the mouth or in liquids without the need to swallow, making them an ideal choice for patients who have difficulty swallowing such as children, the elderly and patients with certain medical conditions. In addition, ODTs have high bioavailability, allowing the drug substance to be quickly delivered into the bloodstream and treatment to begin.
Both well-known, traditional methods of producing tablet dosage forms (granulation, pressing) and innovative, high-tech methods are used to produce ODTs. In a number of studies, encouraging results have been obtained using new technological methods for the preparation of ODTs and new combinations of excipients. 3D-printing, lyophilisation and extrusion technologies seem to be the most promising by now. Among the auxiliaries, various co-process mixtures with a high content of disintegrants are considered to be the most promising.
Among the problems solved by researchers and developers of CCTs are the following: the difficulty of finding an optimal balance between the strength and stability of CCTs and rapid disintegration in the oral cavity; the problem of masking and correcting the taste of CCTs in the case of tablets containing active substances with unpleasant taste or odour; reducing the economic costs of production of CCTs and maximum unification of the technological process with the production of traditional tablets.
Pharmaceutical technology experts continue to work on improving the manufacturing technology of ODTs, searching for new excipients that will provide better stability and bioavailability, as well as new manufacturing methods that will improve product quality and reduce manufacturing costs.
Conflicts of Interest:
Authors report no conflict or competing interest.
References
Kozlova, Zh. M., Pchelincev, S. O., Halikova, E. R., Zabolotnaya, P. G., Maslova, M. N., & Kim, G. A. (2016). The use of modern combined additive agents for directly compressed orodispersible tablets. Drug development & registration, (2), 46-50. (In Russ.).
Mogiljuk, V.V., (2009). Orally disintegrating tablet: biopharmaceutical aspects. Provizor, 1/2: 32–35. (in Rus.).
Brniak, W., Maślak, E., Jachowicz, R., (2015). Orodispersible films and tablets with prednisolone microparticles. Eur J Pharm Sci. Jul 30;75:81-90. View
Kuchekar, B. S., Badhan, A. C., & Mahajan, H. S. (2003). Mouth dissolving tablets: A novel drug delivery system. Pharma times, 35(1), 7-9. View
Korzhavykh, E. A., & Rumyantsev, A. S. (2003). Tablets and their varieties. Rossijskie apteki, 13, 12-16. (In Russ.).
Astha, V., Deepak, B., & Vaminee, M. (2010). Orally disintegrating tablet: boon for market and franchises: a review. International Journal of Drug Formulations & Research, 1, 55 64.
Bhowmik, D., Chiranjib, B., Krishnakanth, P., & Chandira, R. M. (2009). Fast dissolving tablet: an overview. Journal of chemical and pharmaceutical research, 1(1), 163-177. View
Sizyakov, S. A., Alekseev, K. V., Sul'din, A. S., & Alekseeva, S. K. (2008). Modern excipients in direct pressing technology. Farmaciya, 4, 52-56. (In Russ.).
Preethi, S., Selviarun, K., Jagadeesh, S., Nagendre, R., & Prashanth Kumar, M. R. (2013). Oral dispersible tablet–an overview. Int. J. Inv. Pharm. Sci, 1(5), 438-447.
Şenel, S., & Comoglu, T., (2018). Orally disintegrating tablets, fast-dissolving, buccal and sublingual formulations. Pharmaceutical Development and Technology, 23(5), 431. View
Gupta, A. K., Mittal, A., & Jha, K. K. (2012). Fast dissolving tablet-A review. The pharma innovation, 1(1), 1-8. View
Seager, H. (1998). Drug-delivery products and the Zydis fast-dissolving dosage form. Journal of pharmacy and pharmacology, 50(4), 375-382. View
Wiedey, R., Kokott, M., Breitkreutz, J., (2021). Orodispersible tablets for pediatric drug delivery: current challenges and recent advances. Expert Opin Drug Deliv. Dec;18(12):1873-1890. View
Hodzhava, M. V., Demina, N. B., Skatkov, S. A., & Kemenova, V. A. (2011). Effect of slipping agents on the quality of tablet medicines. Farmaciya, 7, 31-33. (In Russ.).
Kaushik, D., (2004). Mouth Dissolving Tablets: A review. / Kaushik, D, Dureja, H, Saini, T. R.// Indian Drugs. – Vol. 41, №4. – R. 187–193. View
Alekseev, K. V., Kedik, S. A., Blynskaya, E. V., Alekseev, V. K., & Maslennikova, N. V. (2015). Pharmaceutical technology. Tablets: Textbook. Ed. by S.A. Kedik. M: CJSC IFT.
Omel'chenko, I. O., Yarnyh, T. G., Borshchevskij, G. I. (2016). Selection of excipients for production of sublingual tablet “Corvalol” by direct pressing method. (In Russ.).
Gordon, M. S., Rudraraju, V. S., Dani, K., & Chowhan, Z. T. (1993). Effect of the mode of super disintegrant incorporation on dissolution in wet granulated tablets. Journal of pharmaceutical sciences, 82(2), 220-226. View
Tranová T, Pyteraf J, Kurek M, Jamróz W, Brniak W, Spálovská D, Loskot J, Jurkiewicz K, Grelska J, Kramarczyk D, Mužíková J, Paluch M, Jachowicz R., (2022). Fused Deposition Modeling as a Possible Approach for the Preparation of Orodispersible Tablets. Pharmaceuticals (Basel). Jan 5;15(1):69. View
Oliveira, L. J., Veiga, A., Stofella, N. C. F., Cunha, A. C., da Graça, T., Toledo, M., Andreazza, I. F., Murakami, F. S., (2020). Development and Evaluation of Orodispersible Tablets Containing Ketoprofen. Curr Drug Deliv. 17(4):348-360. View
Tranová, T., Loskot, J., Navrátil, O., Brniak, W., Mužíková, J., (2023). Effect of co-processed excipient type on properties of orodispersible tablets containing captopril, tramadol, and domperidone. Int J Pharm. Apr 5;636:122838. doi: 10.1016/j. ijpharm.2023.122838. Epub 2023 Mar 13. PMID: 36921743. View
Pyteraf, J., Jamróz, W., Kurek, M., Bąk, U., Loskot, J., Kramarczyk, D., Paluch, M., Jachowicz, R., (2023). Preparation and advanced characterization of highly drug-loaded, 3D printed orodispersible tablets containing fluconazole. Int J Pharm. Jan 5;630:122444. View
Mahesparan, V. A., Bin Abd Razak, F. S., Ming, L. C., Uddin, A. H., Sarker, M. Z. I., & Bin, L. K. (2020). Comparison of Disintegrant-addition Methods on the Compounding of Orodispersible Tablets. International journal of pharmaceutical compounding, 24(2), 148-155. View
Subramanian, S., Sankar, V., Manakadan, A. A., Ismail, S., & Andhuvan, G. (2010). Formulation and evaluation of cetirizine dihydrochloride orodispersible tablet. Pakistan journal of pharmaceutical sciences, 23(2), 232-236. View
Emshanova, S. V., (2008). Methodological approaches to selecting auxiliary substances for the production of tabletized pharmaceutical preparations by direct pressing method. Khimiko-Farmatsevticheskii Zhurnal, 42(2), 38-43. (In Russ.). View
Voskobojnikova, I. V., Avakyan, S. B., Sokol'skaya, T. A., Tyulyaev, I. I., Bagirova, V. L., Kolhir, V. K., & Sakovich, G. S. (2005). Modern auxiliary substances in manufacture of tablets. Use of high-molecular substances for perfection medicinal forms and optimization of technological process. Khimiko Farmatsevticheskii Zhurnal, 39(1), 22-28. (In Russ.). View
Zhilyakova, E. T., Popov, N. N., Novikova, M. Yu., Novikov, O. O., Halikova, M. A., & Lebedeva, O. E. (2011). Study of physico-chemical characteristics of potato starch and corn starch to create prolonged dosage forms with liquid dispersion medium. Actual problems of medicine. Aktual'nye problemy mediciny, 13(4 (99)), 98-105. (In Russ.).
Kuzina, L., Kuzmina, L., & Lukin, N. (2020). Starch and Sugar-containing Substances Use in the Auxiliary Ingredients Complex of Russian and Foreign Pharmaceutical Manufacturers (Comparative Analysis). Bulletin of Science and Practice, 6(11), 132-141. (in Russ.). View
Shimko, O. M., & Hishova, O. M. (2010). The influence of concentration of lactose on the technological properties of a grass potentilla alba. Vestnik farmacii, (3 (49)), 85. (In Russ.).
Peters, H. D. V., & Domo, F. F. (2008). Lactose for direct pressing. Farmacevticheskie tekhnologii i upakovka, (4), 58-60. (In Russ.).
Brys, K. R. F., & Meeus, L. M. F. (2010). U.S. Patent Application No. 12/671,455.
Pavlov, I. N., & Kunichan, V. A. (1999). Grinding of microcrystalline cellulose in the drying process. Khimija Rastitel’nogo Syr’ja, (2), 159-162. (In Russ.).
Lahdenpää, E., Niskanen, M., & Yliruusi, J. (1996). Study of some essential physical characteristics of three Avicel® PH grades using a mixture design. European journal of pharmaceutics and biopharmaceutics, 42(3), 177-182. View
Podczeck, F., & Sharma, M. (1996). The influence of particle size and shape of components of binary powder mixtures on the maximum volume reduction due to packing. International Journal of Pharmaceutics, 137(1), 41-47. View
Williams, R. O., Sriwongjanya, M., & Barron, M. K. (1997). Compaction properties of microcrystalline cellulose using tableting indices. Drug development and industrial pharmacy, 23(7), 695-704. View
Delalonde, M., Bataille, B., Baylac, G., Maurice, J., & Sabatier, R. (1997). Definition of indices for the mechanical design of wet powders: Application to the study of a natural polymer, microcrystalline cellulose. International journal of pharmaceutics, 146(2), 159-165. View
Jerwanska, E., Alderborn, G., Börjesson, E., Newton, J. M., & Nyström, C. (1997). Effect of water content on tensile fracture force and deformability of ram extruded cylinders. International journal of pharmaceutics, 149(1), 131-136. View
Inghelbrecht, S., & Remon, J. P. (1998). Roller compaction and tableting of microcrystalline cellulose/drug mixtures. International journal of pharmaceutics, 161(2), 215-224. View
Teslenko, V. G., Popov, A. A., & Ignatyuk, A. V. (2022). Application of polyvinylpyrrolidone in pharmacy. Lesnoj i himicheskij kompleksy-problemy i resheniya, 482-484. (In Russ.).
Dmitrieva, E. V. (2010). Effect of aerosil on the technological characteristics of tableting masses and tablets. Aspirantskie chteniya, 234-236. (In Russ.).
Kasymov, I. D., & Basevich, A. V. (2021). Study of the technological properties of excipients in the development of the composition of orally dispersible tablets. Drug development & registration. 10(4), 46-53. (In Russ.). View
Bykovskiy S.N., Vasilenko I.A., Demina N.B., Shokhin I.Ye., Novozhilov O.V., Meshkovskiy A.P., & Spitskiy O.R. (2015). Farmatsevticheskaya razrabotka: kontseptsiya i prakticheskiye rekomendatsii. Nauchno-prakt. ruk-vo dlya farmatsevticheskoy otrasli [Pharmaceutical Development: Concept and Practical Guidelines. Scientifi c and practical guide for the pharmaceutical industry]. Moscow: Pero Publ. 472. (In Russ.).
European Pharmacopoeia Commission, & European Directorate for the Quality of Medicines & Healthcare. (2010). European pharmacopoeia (Vol. 1). Council of Europe. View
Mahrous, G. M., Ibrahim, M. A., Mostafa, H. F., & Elzayat, E. M. (2019). Application of a quality-by-design approach for utilizing sodium stearyl fumarate as a taste-masking agent in dextromethorphan hydrobromide orally disintegrating tablets. Pharmaceutical Development and Technology, 24(6), 711-719. View
Van der Merwe, J., Steenekamp, J., Steyn, D., & Hamman, J. (2020). The role of functional excipients in solid oral dosage forms to overcome poor drug dissolution and bioavailability. Pharmaceutics, 12(5), 393. View