Journal of Public Health Issues and Practices Volume 7 (2023), Article ID: JPHIP-219
https://doi.org/10.33790/jphip1100219Research Article
Cardiovascular Responses in Physically Elderly Active People Living with HIV
Martín G. Rosario, Ph.D., PT, CSFI, ATRIC1*, McKenzie Kidwell, SPT2, and Nicole Nelson, SPT3
Texas Woman’s University, Physical Therapy Program, Dallas Campus; Texas, United States.
*Corresponding Author Details: Martín G. Rosario PT, PhD, CSFI, ATRIC, Associate Professor, Texas Woman’s University, Physical Therapy Program, Dallas Campus, 5500 Southwestern Medical Ave. Dallas, TX 75235-7299. United States.
Received date: 13th June, 2023
Accepted date: 25th July, 2023
Published date: 27th July, 2023
Citation: Rosario, M.G., Kidwell, M., & Nelson, N., (2023). Cardiovascular Responses in Physically Elderly Active People Living with HIV. J Pub Health Issue Pract 7(2): 219.
Copyright: ©2023, This is an open-access article distributed under the terms of the Creative Commons Attribution License 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Abstract
Cardiovascular system (CVS) problems are one of the various complications in people living with HIV (PLHIV). In PLHIV, the CVS' capacity to adapt to activities is inadequate.
Purpose: To distinguish the responses of the CVS to a step test in physically active PLHIV.
Results: Significant differences between resting HR and HR after the step test were identified, while there was no significant difference between recovery HR and HR at 76% capacity. Additionally, all cardiovascular measurements were significantly higher than the recovery heart rate.
Conclusion: It appears that physically active participants are experiencing some degree of autonomic dysfunction. However, physical activity seems to help slow down common CV effects.
Introduction
The human immunodeficiency virus (HIV) targets the immune system and weakens the body's defense system against harmful infections. There is no cure for HIV; however, early diagnosis and antiretroviral therapy (ART) increase the survival and longevity rates of people living with HIV (PLHIV) [1]. In 2020 and 2021, 1,072,051 and 38.4 million people were living with a diagnosed HIV infection in the United States and the world, respectively [2,3]. Several studies demonstrate a higher prevalence of comorbidities in PLHIV compared to the general population due to premature aging, side effects of ART, and the biological effects of HIV infection [1]. The most common comorbidities include cardiovascular diseases, cancer, diabetes, dyslipidemia, chronic renal disease, hepatitis B, and hepatitis C [1]. Various cardiovascular complications in PLHIV may impact their overall health and quality of life [4]. Cardiovascular disease (CVD) is a prevalent disease among aging PLHIV and a common contributor to high mortality rates [4]. Since PLHIV have a compromised immune system, they are at greater risk of developing CVD, especially with ineffective management of the modifiable risk factors associated with CVD [5]. Common modifiable CVD risk factors include smoking, dyslipidemia, obesity, and a sedentary lifestyle [6].
HIV and CVD risk factors cause abnormal changes to the cardiovascular system, PLHIV has an increased chance of developing CVD. PLHIV should manage modifiable risk factors as often as possible, for instance, with exercise [5]. Many studies have examined the effects of exercise on the cardiovascular system of those with HIV [7-12]. However, limited research determines the cardiovascular responses these individuals may experience in response to cardiovascular exercise. One study investigated responses of PLHIV in response to single limb support tasks in active PLHIV [9]. However, the study only investigated vestibular and proprioceptive alterations during tasks [9]. Another study mentioned that the variety of factors such as lifestyle (smoking) and sedentary behaviors with a further compromised immune system affects the ability of the cardiovascular system to adapt, illustrated by reduced gait speed and walking distance [13-14]. Rosario and Orozco reported similar outcomes related to age and time of diagnosis in PLHIV [15]. One way to counteract the problems mentioned above is exercise, which is essential for all individuals, especially those with HIV. Exercise helps decrease the virus's detrimental consequences on the cardiovascular system and slow its progression [8]. For instance, Orozco and Rosario found that participation in a long-term exercise program for PLHIV is essential to improve cardiovascular fitness and immune function [8]. The study results showed that long-term aerobic exercise decreased the participant's submaximal heart rate, a positive response to exercise.
As mentioned above, HIV has detrimental effects on the cardiovascular system [7]. However, exercise has positive outcomes in those living with HIV [5]. What is unclear is what are the cardiovascular responses in responses to a submaximal cardiovascular test in PLHIV currently active. Therefore, based on this remark, our study aims to determine the cardiovascular responses to a step test in physically active PLHIV. The current study speculates that although physically active, those living with HIV would exhibit inadequate cardiovascular responses, such as minimal heart rate changes with exercise activities.
Methods
This research was approved by and followed the privacy and confidentiality standards of the La Perla de Gran Precio (LPGP) and IRB (#20092). This study was conducted at the above mentioned location, a community HIV Latino/Hispanic Rehabilitation Clinic, called LPGP in San Juan, Puerto Rico. Prerequisites to participate in this study included having informed consent to access to their CD4+ cell count, HIV status and medical records. The specific inclusion criteria entailed: 1) CD4 levels above 300, 2) walking without an assistive device, 3) tolerating standing position for at least 60 minutes, and 4) ability to go up a flight of stairs without stopping or losing balance. The exclusion criteria included: 1) diagnosis of AIDS (CD4 levels less than 200), Diabetes, Dementia or Arthritis, 2) severe neuropathy, balance impairments, or untreated visual acuity problems, 3) BMI > 40, 4) falls during the last 6 months, 5) back or lower extremities injury or surgery during the last 6 months, and 6) the use of medications that causes drowsiness 24 hours previous to intervention.
Each subject was evaluated by interview and review of their medical record for the inclusion and exclusion criteria assessment. Different tests were performed as a screening tool to identify additional limitations or impairments in the different sensory systems.
Participant enrollments required the following from all subjects: a signed informed consent, clearance by a medical doctor and physical therapist, and an exercise assessment to ascertain the baseline fitness level of each subject by a certified personal trainer. We compiled data related to age, CD4 values, sex, and time since HIV diagnosis.
Participants
21 HIV participants, with the average age of 59.9+/- 5.2 years old, signed the informed consent, and were evaluated through inclusion and exclusion criteria by engaging in an interview and through the review of their medical records.
The step test employed in this study was the Queens College Step Test.(www.topendsports.com/testing/tests/step-queens.htm) The Queens College Step Test uses heart rate recovery to estimate the fitness level. For this study, after the participant sat for 3 minutes, heart rate (HR) was collected with a pulse oximeter, and blood pressure (BP) with a digital blood pressure device. Therefore, the resting HR and BP were recorded. The metronome was set at 88 beats per minute (22 steps per min) for women and 96 beats per minute (24 steps per min) for men. Participants were instructed to step to the beat in an up-up-down-down manner on a 16.25 inches / 41.3 cm step stool. After 3 minutes of testing, the participant immediately stopped, and the tester collected data as previously mentioned for 15 seconds. When participants were too fatigued to finish the test, they stopped, and a researcher collected vitals immediately.
Data Analysis
The current study collected data for heart rate during rest and after the step test. The formula used to calculate the VO2 max was: Men: VO2 max (ml ∙ kg−1 ∙ min−1) = 111.33 - (0.42 × HR) and Women: VO2 max (ml ∙ kg−1 ∙ min−1) = 65.81 - (0.1847 × HR) in accordance with the previously published study by W.D. McArdle et al. (1972). After, data and percentile rank by age and sex were recorded after the predicted VO2 max was calculated using the HR recovery in the equations above. Investigators distinguished 6 data points in this study, heart rate at rest, 64%, 76%, 93% max HR, and recovery. This study used SPSS (version 28) with repeated measures ANOVA to compare resting heart rate with all data points and the recovery heart rate with all data points. We consider a p-value of < 0.05 statistically significant.
Results
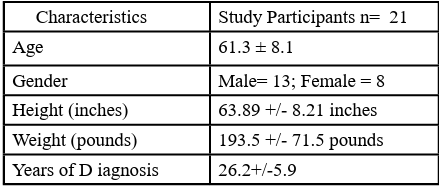
Table 1 illustrates the study sample containing 21 participants. (8 females and 13 males) with a mean age of 61.3 ± 8.1. Additionally, the average weight of participants was 168.03 ± 26.48 pounds, with an average height of 63.89 ± 8.21 inches. Participants' average BMI was calculated at 29.7 kg/m2. Other demographic averages included: a resting HR of 75.38 ± 14.52 beats per minute (bpm), max HR of 158.67 ± 8.11 bpm, and recovery HR of 125.24 ± 29.95 bpm. Of the included individuals, 13 were on blood pressure medications .
Table 2 illustrates the comparisons of heart rate during rest among heart rate percentages. Significant differences were found between resting heart rate and all other conditions. The resting heart rate was lower than the other conditions. Finally, Table 3 compares recovery heart rate and all other points of measurements. Results reveal no difference between the recovery heart rate (125.2 ± 29.9 bpm) and heart rate at 76% capacity (120.6 ± 6.2 bpm). All the other measurements are significantly higher than the recovery heart rate.
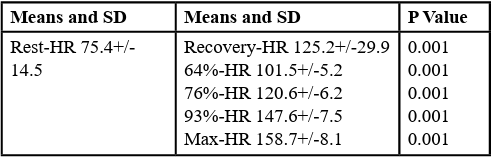
Table 2: Comparisons of heart rate during restresting heart rate among tasks. Results of repeated measures ANOVA performed comparing rest-HR among the other conditions. Significance level set at p≤0.0 5.

Table 3: Comparisons of heart rate during recovery. Results of repeated measure ANOVA performed comparing recovery-HR among the other conditions. Significance level set at p≤0.0 5.
Discussion
This study aimed to examine the cardiovascular responses of PLHIV to a submaximal cardiovascular test. The current study speculated that PLHIV would demonstrate inadequate cardiovascular responses, such as minimal heart rate changes with exercise. However, the results of this study revealed significant differences between resting HR and all other conditions, while there was no significant difference between recovery HR and HR at 76% capacity. Regardless, all cardiovascular measurements were significantly higher than the recovery heart rate. Therefore, we partially accepted our assumption.
Two main discoveries were revealed in this investigation. As illustrated in Table 2, the first outcome suggests that PLHIV can adapt to exercise with increased HR during a step test. Contrary to this study's results, there is substantial evidence related to cardiovascular impairments and inability to acclimate to physical activity commonly seen in PLHIV. A recent review revealed myocardial infarction, heart failure, pulmonary hypertension, sudden cardiac death, and stroke as the most consistent cardiometabolic diseases in PLHIV [15]. In addition, increased sympathetic activity, a biomarker for deteriorating cardiovascular health, was found in PLHIV at rest [16] and after exercise [17]. The heightened risk of cardiovascular complications in PLHIV is not entirely understood. Although advances in treatment strategies have drastically improved the clinical course and life expectancy of those living with HIV, data has raised concerns about an association between ART and CVD risk [18]. Research has also identified the direct effects of HIV, increased inflammation, irregular coagulation, monocyte activation, lifestyle factors, and obesity as significant contributors [19,20].
Despite all the above, this study’s results indicate signs of cardiovascular responses observed during the step test. An explanation is that the current investigation recruited physically active and enrolled in a fitness center. This nonprofit fitness center required subjects to participate in a 3 point exercises program, cardiovascular, strengthening and stretching at least twice a week to be in good standing. The findings in this examination suggest that the participant's activity level correlates with improved CV responses to exercise despite their diagnosis of HIV and use of ART. To our point, Ozemek et al. (2020) reviewed the literature, summarizing the effects of the types and intensities of exercise on cardiorespiratory fitness and cardiometabolic biomarkers [19]. The authors concluded that regular exercise was associated with favorable metabolic profiles and a lower risk of CVD in PLHIV. Additionally, the review found that higher-intensity exercise appeared to result in more significant benefits without exacerbating immune dysfunction [19].
The prevalence of PLHIV aging with a growing burden of CV and metabolic conditions has stimulated significant research interest in this field. Although as mentioned above there is a promising insight of risk factors and the underlying mechanisms driving the process, the exact role of the complex interplay of HIV infection, immune function, ART side effects, autonomic dysfunction, and lifestyle factors on CVD risk need to be explored further, as suggested by Rosario et al. [14]. Future research should also focus on exercise dosage and the effects of regular exercise in different time frames of the disease process.
The second outcome of this study shows that PLHIV has poor cardiovascular response during a step test. This cardiovascular response is illustrated in the difference in resting HR and recovery HR, along with the similarities in recovery HR and the 76% HR data point, as shown in Table 3. The preceding statement implies that the cardiovascular system in these individuals struggles with the restoration phase, since it mimics the HR at ¾ of maximal capacity. Consequently, there was no significant distinction between recovery HR and 76% HR (p-value: 0.54), as brought in Table 3.
Comparable to the above, previous studies have documented that PLHIV has displayed an unusual HR recovery post-cardiovascular exercise [10,12]. Lorenzo et al. found that some participants had an irregular heart rate recovery after performing the exercise treadmill test [10]. This research defined irregular heart rate recovery as a reduction in HR of less than 12 bpm within the first minute of recovery when described concerning HR at peak exercise. According to Lorenzo et al., this inadequate HR recovery response is due to autonomic dysfunction (AD) caused by HIV. Autonomic dysfunction can cause a decreased vagal tone of the heart, leading to an unusual HR recovery after exercise. The current investigation observed a difference greater than 12 bpm; therefore our assumption is similar to that of Lorenzo et al., cardiovascular issues due to AD.
AD presented in PLHIV has been previously documented [10,11]. AD seems more prevalent in those in advanced phases of HIV, but can still be present in earlier stages [11-12] Rosario et al., [23] examine the cardiovascular variations during a sit-to-stand in PLHIV. The authors identified significant cardiovascular changes with limited responses during postural variations further supporting this study's finding. Further, those who have investigated AD in PLHIV proposed that AD could be due to the effects of ART medication [12,14]. Askgaard et al. performed a study evaluating whether AD was present in HIV patients receiving ART and the effects on the cardiovascular system. They discovered that the HIV patient's total heart variability, measured as the standard deviation of normal-to normal, was lower compared to the healthy controls, highlighting the effects of AD on PLHIV [14].
Conclusion
This study aimed to determine the cardiovascular responses in PLHIV. This study revealed that PLHIV could somewhat adapt to exercise, as shown by the increase in HR in response to the rising cardiovascular demand during the step test. The above could be due to the higher activity in the participants compared to other PLHIV who show signs of those suffering heart failure and cardiovascular impairments in response to exercise. In addition, the study demonstrated that PLHIV has abnormal HR recovery after exercise. This previous remark could be explained by the decreased fitness levels in those with HIV compared to healthy individuals, decreased vagal tone, hyperlipidemia, hypertension, and other CVD complications associated with HIV. Other assumptions related to poor cardiovascular responses are related to peripheral neuropathy [24], reduced cd4 count [25], and age [21]. Regardless of the cause, based on this study's findings, we encourage clinicians to consider the above when prescribing exercises to PLHIV, and to monitor HR in order to adjust the dosage accordingly.
We recommend future studies investigate the cardiovascular responses in PLHIV, comparing individuals taking different ART combinations. This approach could give insight into the medication's effects on the cardiovascular system during and after cardiovascular exercise. We also propose that further examinations investigate recovery HR in those with HIV following cardiovascular exercise, with larger sample sizes across all stages of HIV. Having a better view of the impact of HIV in diverse points could help plan PT interventions for those with HIV and give clinicians an idea of what to expect from these patients. Lastly, PTs should be familiar with the abnormal findings concerning PLHIV and their vital signs during and following cardiovascular exercise, along with regularly assessing the cardiovascular capacity of PLHIV.
Declaration
Funding: N/A
Competing interests:
Authors report no conflict or competing interest.
Ethics approval:
IRB approval TWU protocol # 20092
Consent to participate:
The participants gave signed consent for this study
Authors' contributions:
All authors contributed to the study’s conception and design.
References
Roomaney, R. A., van Wyk B, Pillay-van Wyk, V. (2022). Aging with HIV: Increased Risk of HIV Comorbidities in Older Adults. Int J Environ Res Public Health. 19(4):2359. Published 2022 Feb 18. doi:10.3390/ijerph19042359View
Centers for Disease Control and Prevention. (2022, August 26). HIV surveillance. Centers for Disease Control and Prevention. Retrieved October 8, 2022, from https://www.cdc.gov/hiv/ library/reports/hiv-surveillance.html View
World Health Organization. (n.d.).(2022). HIV. World Health Organization. Retrieved October 8, from https://www.who.int/ data/gho/data/themes/hiv-aidsView
Aberg, Judith A MD. (2009). Cardiovascular Complications in HIV Management: Past, Present, and Future. JAIDS Journal of Acquired Immune Deficiency Syndromes: January 1, Volume 50 - Issue 1 - p 54-64. Doi: 10.1097/QAI.0b013e31818ceaa4 View
Falusi, O. M., & Aberg, J. A., (2011). HIV and cardiovascular risk factors. AIDS Read, vol. 11(5) pp. 263-8
Fedele, F., Bruno, N., Mancone, M., (2011). Cardiovascular risk factors and HIV disease. AIDS Rev. Apr-Jun;13(2):119-29. PMID: 21587343.View
Dau, B., Holodniy, M., (2008). The Relationship Between HIV Infection and Cardiovascular Disease. Curr Cardiol Rev. Aug;4(3):203-18. doi: 10.2174/157340308785160589. PMID: 19936197; PMCID: PMC2780822.View
Orozco, E., & Rosario, M.G. (2020). Overall fitness benefits in individuals with HIV participating in a community- based exercise program. J Rehab Pract Res 1(2):109. https://doi. org/10.33790/jrpr1100109View
Rosario, M.G., Corrigan, K., Orozco, E., & Bowman, C., (2022). Single Limb Support Instability combined with Vestibular and Proprioceptive Alteration in Hispanic Latinx Living with HIV. International Journal of Physiotherapy, 9(1), 01-08. https://doi. org/10.15621/ijphy/2022/v9i1/1143View
Lorenzo, A. D., Meirelles, V., Vilela, F., & Souza, F. C. (2012). Use of the exercise treadmill test for the assessment of cardiac risk markers in adults infected with HIV. Journal of the International Association of Providers of AIDS Care (JIAPAC), 12(2), 110–116. https://doi.org/10.1177/1545109712460098View
Roy Freeman, Mark S. Roberts, Lawrences S. Friedman, Christopher Broadbridge. (1990). Autonomic function and human immunodeficiency virus infection. Neurology, 40 (4), 575. https://n.neurology.org/content/40/4/575.shortView
Cade, W. T., Reeds, D. N., Lassa-Claxton, S., Davila-Roman, V. G., Waggoner, A. D., Powderly, W. G., & Yarasheski, K. E. (2008). Post-exercise heart rate recovery in HIV-positive individuals on highly active antiretroviral therapy. Early indicator of cardiovascular disease? HIV Medicine, 9(2), 96 100. https://doi.org/10.1111/j.1468-1293.2007.00524.xView
Orozco, Elizabeth, Rosario, G. Martin. (2022). Risk Factors of Cardiovascular Disease as Predictors of Cardiomotor Profiles in Hispanic-Latinos Living with HIV. International Journal of Physical Education, Fitness and Sports (IJPEFS), 11 (3), 9-20. https://doi.org/10.34256/ijpefs2232View
Rosario, M.G., Gines, G., Jamison, L. (2020). Lifestyle, Physical and Cardiovascular Components Associated with Immune Profile in Hispanic-Latino People Living with HIV. J Ment Health Soc Behav 2(1):121. https://doi.org/10.33790/ jmhsb1100121View
De Francesco, Davidea; Sabin, Caroline A.a; Reiss, Peterb,c. Multimorbidity patterns in people with HIV. Current Opinion in HIV and AIDS 15(2):p 110-117, March 2020. | DOI: 10.1097/ COH.0000000000000595View
McIntosh R. C. (2016). A meta-analysis of HIV and heart rate variability in the era of antiretroviral therapy. Clinical autonomic research : official journal of the Clinical Autonomic Research Society, 26(4), 287–294. https://doi.org/10.1007/s10286-016 0366-6View
Borges, J., Soares, P., & Farinatti, P. (2012). Autonomic modulation following exercise is impaired in HIV patients. International journal of sports medicine, 33(4), 320–324. https://doi.org/10.1055/s-0031-1297954View
Barbaro, G., Fisher, S. D., & Lipshultz, S. E. (2001). Pathogenesis of HIV-associated cardiovascular complications. The Lancet. Infectious diseases, 1(2), 115–124. https://doi. org/10.1016/S1473-3099(01)00067-6View
Ozemek, C., Erlandson, K. M., Jankowski, C. M., (2020). Physical activity and exercise to improve cardiovascular health for adults living with HIV. Prog Cardiovasc Dis. 63(2):178-183. doi:10.1016/j.pcad.2020.01.00View
Dominick, L., Midgley, N., Swart, L.-M., Sprake, D., Deshpande, G., Laher, I., Joseph, D., Teer, E., & Essop, M. F. (2020). HIV-related cardiovascular diseases: The search for a unifying hypothesis. American Journal of Physiology-Heart and Circulatory Physiology, 318(4). https://doi.org/10.1152/ ajpheart.00549.2019View
Rosario, M.G. & Orozco, E., (2021). The Influence of Age on Cardiovascular, Motor, and Lifestyle Components in Hispanic Latinos Living with HIV. J Pub Health Issue Pract 5(2): 190. doi: https://doi.org/10.33790/jphip1100190View
Askgaard, G., Kristoffersen, U. S., Mehlsen, J., Kronborg, G., Kjaer, A., & Lebech, A.-M. (n.d.). Decreased heart rate variability in HIV positive patients receiving antiretroviral therapy: Importance of blood glucose and cholesterol. PLOS ONE. Retrieved April 18, 2023, from https:// journals.plos.org/plosone/article?id=10.1371%2Fjournal. pone.0020196#pone.0020196-Welby1View
Rosario, M. G., and Gonzalez-Sola, M., (2018). Autonomic nervous system assessment in people with HIV: A cross-sectional study [version 1; peer review: 1 not approved]. F1000Research , 7:696 (https://doi.org/10.12688/f1000research.14685.1)View
Rosario, M.G., Jamison, L., Gines, G.,. (2021). Peripheral Neuropathy Impacts Gait Motor Components in Hispanic Latinx living with HIV. Internal Journal of Sports Medicine and Rehabilitation, 4:20. DOI: 10.28933/ijsmr-2021-03-0306View
Rosario, M.G., Jamison L., & Gines G. (2020). The Role of HIV Antiretroviral Medication on Motor-Cognitive and Neurological Alterations in Hispanic People Living with HIV. J Pub Health Issue Pract 4(1):160.https://doi.org/10.33790/jphip1100160 View
W.D. McArdle et al. (1972). Reliability and interrelationships between maximal oxygen uptake, physical work capacity and step test scores in college women. Medicine and Science in Sports, Vol 4, p182-186. View