Journal of Social Work and Welfare Policy Volume 3 (2025), Article ID: JSWWP-158
https://doi.org/10.33790/jswwp1100158Review Article
Epigenetic Implications of Substance Abuse in Latino Populations: A Review of Substance-Specific and Intergenerational Outcomes
Noel Casiano, PsyD, LMFT
1The Life Center of CT, Inc, 15-17 May Street, Hartford, CT 06105, United States.
Corresponding Author Details: Noel Casiano, The Life Center of CT, Inc, 15-17 May Street, Hartford, CT 06105 United States.
Received date: 04th July, 2025
Accepted date: 13th August, 2025
Published date: 16th August, 2025
Citation: Casiano, N., (2025). Epigenetic Implications of Substance Abuse in Latino Populations: A Review of Substance Specific and Intergenerational Outcomes. J Soci Work Welf Policy, 3(2): 158.
Copyright: ©2025, This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The Latino population encompasses unique socio-cultural and environmental nuances that could affect epigenetic alterations, yet these populations are still severely underrepresented in epigenomic studies. Substance abuse significantly alters gene expression through epigenetic mechanisms, including DNA methylation, histone modifications, and microRNA regulation. These changes can lead to long-lasting effects on neurobiological development and behavior, with implications that extend across generations. Among Latino populations, sociocultural stressors such as immigration, poverty, and systemic discrimination intersect with high rates of Adverse Childhood Experiences (ACEs), amplifying vulnerability to substance use and its intergenerational effects. This review synthesizes the current evidence on the epigenetic consequences of various substances—alcohol, nicotine, cannabis, opioids, and stimulants— focusing on their transmission pathways and behavioral outcomes in offspring. It integrates data from rodent models and emerging human studies with a specific emphasis on Latino populations. The findings underscore the need for culturally informed prevention and intervention strategies that consider both biological inheritance and social context. These strategies have the potential to significantly reduce existing health disparities in the Latino population suffering from substance abuse and to reveal exciting potential health benefits of these strategies.
Keywords: Epigenetics, Substance Use Disorder, Latino Populations, Intergenerational Transmission, DNA Methylation, Adverse Childhood Experiences, Transgenerational Inheritance
Introduction
Substance use disorders (SUDs) are multifaceted, chronic conditions that arise from a complex interplay of genetic predisposition, environmental exposure, and neurobiological alterations. Traditionally, research on addiction has emphasized behavioral, cognitive, and pharmacological pathways. However, recent advances in molecular biology have illuminated the epigenetic landscape as a critical mediator in the development and persistence of SUDs. Epigenetics refers to heritable changes in gene expression that occur without modifications to the underlying DNA sequence, primarily through mechanisms such as DNA methylation, histone modification, and non-coding RNA regulation [1]. These molecular alterations can be triggered by exposure to psychoactive substances, resulting in long-lasting effects on brain function, stress reactivity, and behavior.
Compelling evidence now shows that these drug-induced epigenetic changes can persist well beyond the period of substance use, potentially contributing to relapse vulnerability and long-term neuropsychiatric consequences. Even more concerning is the growing body of research indicating that such changes can be transmitted to subsequent generations through epigenetic inheritance, thereby influencing the biological and psychological trajectories of offspring [2,3]. This transgenerational transmission of epigenetic information introduces new dimensions to our understanding of addiction risk and prevention, particularly within families and communities already facing systemic health disparities.
Among the populations most affected by these intersecting factors are Latino communities, who face disproportionate burdens related to substance use and mental health outcomes. These disparities are often compounded by culturally specific stressors, including immigration-related trauma, intergenerational family separation, discrimination, language barriers, and chronic socioeconomic instability [4]. Such experiences are associated with higher rates of Adverse Childhood Experiences (ACEs), which have been shown to significantly influence epigenetic regulation. Particularly in stress related gene pathways such as NR3C1, BDNF, and FKBP5. This has been shown to increase vulnerability to substance use initiation and escalation [5,6].
Despite the high prevalence of ACEs and substance-related challenges in Latino populations, these groups remain grossly underrepresented in epigenetic research, particularly studies examining the biological impacts of environmental stressors and drug exposure. Most current epigenetic findings are based on predominantly White, non-Hispanic cohorts, limiting the generalizability and cultural relevance of the conclusions drawn. This omission contributes to a broader gap in personalized, equitable care and perpetuates health disparities in prevention and treatment outcomes for Latino individuals with SUDs.
This review seeks to address that gap by providing a comprehensive synthesis of the current research on how various substances such as alcohol, opioids, and stimulants, alter epigenetic mechanisms, and how these alterations might contribute to long-term and intergenerational consequences in vulnerable populations. Specifically, this paper explores the epigenetic implications of substance use in Latino populations, highlighting the intersection of biological vulnerability and socio-environmental stress, and advocating for more inclusive research frameworks that can inform culturally tailored interventions and policy reforms.
Methodology
This narrative review employed a systematic search of the literature from 2010 to 2025 using electronic databases, including PubMed, PsycINFO, and Scopus. Search terms included "epigenetics," "substance abuse," "Latino populations," "intergenerational transmission," "DNA methylation," "histone modification," "miRNA," and "ACEs." Studies were included if they examined epigenetic changes due to substance exposure in either human Latino populations or animal models relevant to human physiology. Articles that focused on intergenerational or transgenerational effects, particularly those exploring behavioral and cognitive outcomes in offspring, were prioritized. Additional sources were identified through reference lists of key studies.
The initial search yielded 3,217 articles. After screening for relevance and duplication, 112 articles were reviewed in full, and 36 met all inclusion criteria. Of these, 18 involved rodent models demonstrating specific epigenetic mechanisms, 10 were longitudinal or cohort studies with Latino participants, and 8 were systematic reviews or meta-analyses relevant to intergenerational transmission.
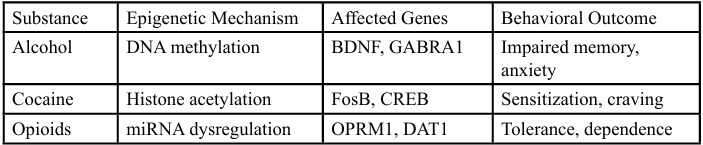
To provide a clearer picture of substance-specific emphasis, we include the following figures:
Discussion of Figures
The data presented in Figure 1 provides a concise overview of the diverse epigenetic mechanisms influenced by different substances of abuse. Alcohol, for example, has been shown to exert its neurobiological impact primarily through DNA methylation, particularly at gene promoters associated with neuroplasticity and inhibitory signaling such as BDNF and GABRA1. Studies have consistently demonstrated that alcohol-induced hypermethylation of BDNF leads to reduced gene expression and diminished synaptic plasticity, contributing to cognitive impairments and heightened anxiety-like behaviors in both animal models and humans [7,8].
Cocaine, in contrast, is associated with alterations in histone acetylation, which influence chromatin structure and gene transcription. Research by Robison and Nestler [9] and Heller et al. [10] highlights the role of increased acetylation at the promoters of FosB and CREB, two key transcription factors involved in reward processing and long-term behavioral adaptations. These changes are linked to drug-induced sensitization, enhanced reinforcement, and the compulsive nature of cocaine use.
Opioid exposure appears to primarily disrupt the expression of microRNAs (miRNAs)—non-coding RNAs that post transcriptionally regulate gene expression. Notably, changes in miRNAs targeting OPRM1 (the mu-opioid receptor gene) and DAT1 (dopamine transporter) have been observed in both preclinical and human studies [11,12]. These disruptions can modulate dopamine and opioid receptor signaling, leading to neuroadaptive changes that underpin tolerance, withdrawal, and dependence.
The divergent epigenetic signatures identified across substances reinforce the concept that addiction is not a monolithic disorder, but rather a collection of substance-specific syndromes with distinct biological substrates. As such, personalized medicine approaches that consider the epigenetic profiles induced by particular drugs, could lead to more targeted and effective treatment modalities [1,13].
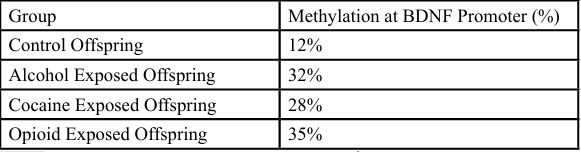
Figure 2 provides compelling evidence for the heritability of substance-induced epigenetic modifications. The increased methylation levels at the BDNF promoter in the offspring of rodents exposed to alcohol, cocaine, and opioids suggest that these epigenetic changes are not confined to the directly exposed individual but can be transmitted across generations. Such findings align with a growing body of research indicating that parental substance use can result in germline epigenetic alterations that affect offspring neurodevelopment and behavior [2,3].
Specifically, the elevated methylation observed in opioid-exposed offspring (35%) is particularly noteworthy, as it may indicate a more persistent or robust transmission of epigenetic changes. BDNF methylation is associated with impairments in hippocampal function, stress reactivity, and memory consolidation, all of which are implicated in the etiology of addiction and comorbid psychiatric conditions [5]. The intergenerational effects of substance use thus raise significant public health concerns, especially for populations with high rates of familial substance abuse.
From a clinical perspective, these findings underscore the necessity of integrating reproductive and perinatal care into addiction treatment strategies. Counseling patients on the potential transgenerational impacts of substance use, especially during pregnancy, could support both prevention efforts and therapeutic interventions for at risk youth. Furthermore, the reversibility of these epigenetic marks remains a critical avenue for research. Animal studies suggest that interventions such as environmental enrichment or pharmacological agents (e.g., HDAC inhibitors) can partially restore gene expression profiles in offspring [14,15].
Collectively, Figures 1 and 2 reveal a complex but coherent epigenetic narrative. Substances of abuse induce lasting biological changes that not only affect the user but may also influence the genetic and neurobehavioral outcomes of their descendants. These findings advocate for the integration of epigenetic screening tools in addiction medicine, and for further exploration into therapies aimed at reversing or mitigating these changes at the molecular level.
Latino-Specific Findings Studies focusing on Latino populations reveal disproportionate exposure to ACEs, which correlate strongly with early initiation and escalation of substance use. Sánchez Rivera et al. [16] found that Latino adolescents with four or more ACEs showed significantly higher rates of tobacco (OR = 1.49), alcohol (IRR = 1.23), and marijuana use (IRR = 1.66). These behavioral outcomes suggest a pathway in which early adversity leads to neurobiological vulnerability, mediated by epigenetic modifications. Furthermore, Blackson et al. [17] demonstrated that paternal substance use history among Latino immigrants is a significant predictor of SUD risk in their children, emphasizing the relevance of intergenerational transmission even when exposure occurs pre-immigration.
Discussion
The reviewed evidence supports the hypothesis that substance induced epigenetic changes contribute to behavioral and psychological vulnerabilities that are passed across generations. These changes occur in genes regulating stress response (e.g., NR3C1), neuroplasticity (e.g., BDNF), and reward pathways (e.g., DRD2). For Latino families, this transmission is compounded by environmental factors such as chronic stress, discrimination, and limited access to mental health resources.
Animal studies offer mechanistic insights, showing how maternal or paternal drug exposure can alter epigenetic markers in offspring. For instance, paternal nicotine exposure in rodents alters histone methylation patterns and miRNA expression in the sperm, leading to anxiety-like behaviors in F1 and F2 generations. Cannabis exposure affects methylation in genes related to synaptic function, correlating with impaired memory and social interaction in offspring. While human studies are still developing, preliminary findings suggest similar patterns. The alignment of behavioral data from Latino populations with known epigenetic pathways highlights the importance of integrating genetic, developmental, and cultural factors into prevention strategies. Interventions should focus on reducing ACEs and supporting family systems to mitigate these epigenetic risks.
Limitations
While this review offers important insights into the epigenetic consequences of substance use and their potential intergenerational impacts, particularly within Latino populations, it is critical to acknowledge several limitations that constrain the current body of evidence.
First, there is a significant lack of epigenomic research specifically focused on Latino populations, which severely limits the cultural specificity, generalizability, and translational potential of existing findings. Most epigenetic studies in addiction research are based on predominantly White, non-Hispanic samples, creating a substantial blind spot in our understanding of how substance use interacts with the unique sociocultural and historical experiences of Latino individuals. This underrepresentation hinders the development of culturally responsive prevention and treatment strategies and perpetuates health disparities in addiction care.
Second, much of the mechanistic evidence regarding epigenetic alterations comes from preclinical animal models. While these studies provide critical insights into the molecular pathways affected by substances of abuse, they may not fully capture the complexity of human epigenetics, particularly in the context of polygenic influences, diverse environmental exposures, and dynamic social determinants of health. Rodent models, for instance, may not replicate the intricate gene-environment interactions or developmental trajectories observed in human populations, limiting the external validity of these findings.
Third, a large portion of human studies in this area rely on cross sectional designs, which inherently restrict the ability to establish causal relationships between substance use, epigenetic modifications, and behavioral outcomes. These designs also make it difficult to differentiate between direct epigenetic effects of substance exposure and inherited (transgenerational) epigenetic changes passed on from previous generations. Longitudinal studies are needed to disentangle these pathways and to examine how early-life adversity, timing of exposure, and duration of substance use shape epigenetic trajectories over time.
Fourth, socioeconomic, cultural, and contextual confounders, such as acculturative stress, systemic racism, educational disparities, and access to care, are frequently under-measured or omitted in current research. These unaccounted variables may obscure or distort the true magnitude and nature of epigenetic risk factors in Latino populations, leading to imprecise or biased risk estimates. Integrating mixed methods approaches and community-engaged research designs could help capture these nuanced influences and improve the ecological validity of future studies.
Finally, technical and methodological variability across epigenetic studies, including differences in tissue types, analytical platforms, and data processing pipelines, further complicates synthesis and replication. Standardized protocols and more inclusive sampling frameworks will be essential to advancing the field in a direction that is both biologically rigorous and socially equitable.
Opportunities for Future Research
Despite growing interest in the epigenetic underpinnings of substance use and addiction, significant gaps remain, particularly in research that centers Latino populations. This section outlines critical avenues for future investigation to advance both scientific understanding and culturally responsive intervention strategies.
1. Advancing Epigenetic Research in Latino Populations
One of the most pressing needs is the inclusion of Latino populations in epigenomic research. These communities remain underrepresented in large-scale genomic studies, limiting the generalizability and applicability of current findings.
• Underrepresentation and Sociocultural Stressors: Future research should examine how unique sociocultural stressors, such as immigration-related trauma, discrimination, acculturative stress, and chronic poverty, interact with substance-induced epigenetic modifications.
• Culturally Specific Epigenetic Pathways: There is also a need to determine whether Latino-specific environmental exposures influence epigenetic regulation differently than in other racial and ethnic groups, potentially identifying unique biological pathways of risk and resilience.
2. Longitudinal and Multigenerational Designs
To unravel the causal and temporal dimensions of epigenetic alterations, longitudinal and multigenerational studies are essential.
• Causal Mechanisms: Longitudinal research can help clarify causal pathways linking substance use to epigenetic modifications and subsequent behavioral or psychiatric outcomes in offspring.
• Critical Windows of Exposure: Investigating the timing (e.g., prenatal, perinatal, adolescence) and duration of substance exposure will provide insight into sensitive developmental periods that may shape epigenetic trajectories and transgenerational inheritance.
3. Reversibility and Intervention Potential
A promising area of exploration is the potential reversibility of substance-induced epigenetic changes, opening doors to novel prevention and treatment approaches.
• Therapeutic Targets: Research should explore whether pharmacological agents (e.g., histone deacetylase inhibitors), environmental enrichment, or psychosocial interventions can reverse maladaptive epigenetic modifications.
• Preventive Interventions: Early-life interventions, particularly those administered during pregnancy or early childhood, may buffer against adverse transgenerational effects and should be rigorously evaluated.
4. Substance-Specific Mechanistic Studies
Greater specificity is needed in understanding how different substances uniquely affect epigenetic profiles.
• Substance Variability: Comparative studies examining the epigenetic effects of alcohol, opioids, cannabis, and stimulants could help identify substance-specific biological mechanisms and behavioral phenotypes.
• Gene-Level Investigations: Focused research on genes implicated in addiction and stress regulation (e.g., BDNF, NR3C1, OPRM1) may shed light on the molecular pathways linking substance exposure to neurodevelopmental and behavioral outcomes.
5. Sociocultural and Environmental Moderators
A deeper integration of sociocultural context is vital to understanding how structural and interpersonal factors shape epigenetic vulnerability.
• Adverse Childhood Experiences (ACEs): There is a need to examine how cumulative ACEs, particularly within Latino families, influence epigenetic sensitivity to substance use and co-occurring mental health disorders.
• Structural Inequities: Research should explore how systemic racism, housing insecurity, and economic instability contribute to epigenetic dysregulation and addiction risk.
6. Community-Based Participatory and Mixed-Methods Research
To ensure cultural relevance and ethical soundness, future research must adopt participatory and integrative approaches.
• Community Engagement: Studies should prioritize frameworks that actively involve Latino families in all stages of the research process, from study design to dissemination, to foster trust and cultural alignment.
• Mixed-Methods Integration: Combining quantitative epigenetic data with qualitative insights can provide a holistic understanding of lived experiences and environmental exposures that contribute to addiction risk.
7. Mechanisms of Intergenerational Transmission
The biological and behavioral pathways by which substance-related epigenetic modifications are transmitted across generations remain underexplored.
• Parental Transmission Patterns: Future studies should differentiate between maternal and paternal transmission of epigenetic alterations and their respective impacts on offspring development.
• Neurobehavioral Outcomes: Research is needed to connect inherited epigenetic changes to specific behavioral and cognitive traits, such as stress reactivity, learning, memory, and substance use susceptibility.
8. Policy and Intervention Development
Epigenetic findings have the potential to inform the development of culturally grounded prevention and intervention programs, as well as public health policies.
• Culturally Tailored Interventions: There is an urgent need to translate epigenetic research into effective interventions that are responsive to the cultural values, stressors, and strengths of Latino families.
• Policy Implications: Evidence from epigenetic studies can support advocacy for systemic reforms aimed at reducing disparities in addiction treatment access, quality, and outcomes.
9. Development of Epigenetic Screening and Predictive Tools
Applied research can facilitate the creation of screening tools to identify individuals at heightened risk for substance use disorders.
• Diagnostic Applications: Identifying reliable epigenetic biomarkers may enable early detection of vulnerability to addiction, allowing for timely intervention.
• Integrated Predictive Models: Future studies should develop risk models that incorporate epigenetic data alongside ACEs and sociocultural variables to improve predictive accuracy and precision medicine approaches.
10. Cross-Population and Global Comparisons
Finally, cross-ethnic and cross-national studies can contextualize findings and reveal universal versus population-specific mechanisms.
• Ethnic Comparisons: Comparative analyses across racial and ethnic groups can help identify both shared and culturally distinct epigenetic pathways associated with substance use.
• Global Perspectives: Research should explore how sociocultural stressors, such as displacement, discrimination, and poverty, impact epigenetic mechanisms of addiction in other marginalized populations globally, enhancing cross-cultural understanding and solidarity.
Practice and Policy Implications
The implications of these findings for social work and substance abuse treatment are far-reaching, demanding changes in both practice and policy. In clinical settings, social workers and substance use professionals must integrate an understanding of epigenetic processes into assessment and intervention, recognizing that substance exposure can produce enduring molecular changes that influence health across generations. This requires a shift toward trauma-informed, culturally responsive care that addresses not only the biological consequences of substance use, but also the layered social determinants such as immigration trauma, acculturative stress, racial discrimination, and economic marginalization, that shape vulnerability within Latino communities. Practice models should emphasize family-centered and multigenerational approaches, accounting for both maternal and paternal influences on intergenerational health, while leveraging cultural strengths as protective factors. Workforce development initiatives must also expand training on the biopsychosocial mechanisms of addiction risk, equipping practitioners to address the intersection of biological, psychological, and social dimensions of recovery.
From a policy perspective, these insights highlight the urgent need for investment in early childhood interventions, parental support, and school-based prevention programs that are informed by the understanding that the timing and intensity of interventions can influence epigenetic expression and long-term outcomes. Federal, state, and local funding mechanisms should prioritize culturally grounded, community-driven programs that respond to the lived realities of Latino populations and other historically underrepresented groups. Policy must also ensure that ethnically diverse populations are meaningfully included in federally funded biomedical and behavioral research, thereby closing gaps in epigenetic knowledge and ensuring findings are generalizable. Furthermore, coordinated policies should incentivize interdisciplinary collaboration between molecular biologists, social scientists, public health professionals, and community organizations to develop integrated solutions. Addressing the root causes of substance use, poverty, housing insecurity, discrimination, and limited access to care remains essential to preventing the environmental stressors that activate harmful epigenetic pathways. Embedding these biological insights into the design of prevention, intervention, and recovery strategies holds transformative potential for reducing health disparities, breaking intergenerational cycles of adversity, and fostering resilience in Latino populations and beyond.
Conclusion
The evidence reviewed in this article highlights the profound impact of substance abuse on the epigenome, demonstrating that drug exposure can induce enduring molecular changes that influence gene expression, neural development, and behavior. These epigenetic alterations not only affect individuals during their lifetime but may also be transmitted to their offspring, thereby perpetuating biological vulnerability across generations. Such findings underscore the role of epigenetics as a crucial mechanism linking environmental exposure to long-term health and psychological outcomes.
Within Latino populations, the consequences of substance use intersect with culturally specific experiences and systemic inequities, such as immigration trauma, acculturative stress, racial discrimination, and economic marginalization to create layered and compounded risk. These factors contribute to higher rates of Adverse Childhood Experiences (ACEs) and reduced access to culturally responsive care, conditions that may further exacerbate epigenetic susceptibility to addiction and mental health disorders. The intersection of these biological and social dimensions demands a paradigm shift in how substance use disorders are studied, understood, and addressed within underrepresented populations.
To break these cycles of adversity, there is an urgent need for interdisciplinary research that bridges molecular biology, developmental psychology, public health, and social justice. Future studies must prioritize longitudinal, multigenerational designs that can distinguish between direct and inherited epigenetic effects, and they must include ethnically and culturally diverse cohorts, especially Latino populations, who have historically been excluded from biomedical research. Additionally, integrating community based participatory research methods can ensure that study designs are culturally grounded, ethically sound, and attuned to the lived realities of marginalized populations.
Equally important is the identification of reversible epigenetic markers that may serve as targets for intervention. Emerging therapies such as epigenetic editing, pharmacological demethylation agents, and environmental enrichment programs, offer promising avenues for mitigating the long-term effects of substance exposure. However, for these approaches to be truly effective, they must be coupled with interventions that also address the social determinants of health that shape risk and resilience across generations.
To translate the insights of epigenetic research into meaningful change for vulnerable populations, it is essential that policymakers, program designers, and service providers integrate these findings into the structures that shape health and behavioral outcomes. Epigenetics offers a compelling biological lens through which to understand the long-term effects of social determinants such as poverty, discrimination, immigration trauma, and adverse childhood experiences. These insights underscore the urgent need for trauma informed, culturally responsive, and prevention-focused policies that acknowledge how environmental adversity can become biologically embedded across generations.
From a social policy perspective, investments in early childhood support programs, mental health access, and substance use prevention must be informed by the understanding that the timing and intensity of interventions can influence epigenetic expression and outcomes. Program design should prioritize family-centered models that account for both maternal and paternal influences on intergenerational health. Furthermore, professional practice across social work, education, and behavioral health must be adapted to include training in the biopsychosocial mechanisms of addiction risk, empowering practitioners to respond more effectively to the communities they serve.
By embedding epigenetic insights into multi-level strategies, from federal funding mechanisms to frontline service delivery, professionals can begin to disrupt the biological cycles of trauma, inequality, and substance use that disproportionately affect Latino populations and other historically marginalized populations. This translational bridge between science and service holds transformative potential for reducing health disparities and promoting resilience across generations.
In summary, understanding the epigenetic consequences of substance abuse, particularly within Latino populations, offers a unique opportunity to develop more equitable, holistic, and biologically informed strategies for addiction prevention, intervention, and recovery. Addressing the intergenerational legacy of addiction will require nothing less than a coordinated, culturally responsive, and scientifically rigorous approach that honors both the molecular and human dimensions of healing.
Competing Interests:
The author declares that he has no competing interests.
References
Nestler, E. J. (2016). Transgenerational epigenetic contributions to stress responses. Nature Reviews Neuroscience, 17(6), 309 317. View
Vassoler, F. M., & Sadri-Vakili, G. (2014). Mechanisms of transgenerational inheritance of addictive-like behaviors. Neuroscience, 264, 198–206. View
Skvortsova, K., Iovino, N., & Bogdanović, O. (2018). Functions and mechanisms of epigenetic inheritance in animals. Nature Reviews Molecular Cell Biology, 19(12), 774–790. View
Alegría, M., Alvarez, K., Ishikawa, R. Z., DiMarzio, K., & McPeck, S. (2017). Removing obstacles to eliminating racial and ethnic disparities in behavioral health care. Health Affairs, 35(6), 991–999. View
McGowan, P. O., Sasaki, A., D’Alessio, A. C., Dymov, S., Labonté, B., Szyf, M., ... & Meaney, M. J. (2009). Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neuroscience, 12(3), 342–348.View
Blaze, J., & Roth, T. L. (2015). Evidence from clinical and animal model studies of the long-term and transgenerational impact of stress on DNA methylation. Seminars in Cell & Developmental Biology, 43, 76–84. View
Sakharkar, A. J., Zhang, H., Tang, L., & Pandey, S. C. (2012). Histone Deacetylases (HDAC)-Induced Histone Modifications in the Amygdala: A Role in Rapid Tolerance to the Anxiolytic Effects of Ethanol. Alcohol Clin Exp Res, Vol 36, No. 1; 61-71. View
Domi, E., Domi, A., Adermark, L., Heilig, M., Augier, E. (2021). Neurobiology of alcohol seeking behavior. Journal of Neurochemistry. 157 (5). 1585-1614. View
Robison, A. J., & Nestler, E. J. (2011). Transcriptional and epigenetic mechanisms of addiction. Nature Reviews Neuroscience, 12(11), 623–637. View
Maze, Ian & Nestler, Eric J. (2011). The epigenetic landscape of addiction. Annals of the New York Academy of Sciences. 1216 (1). 99-113. View
Zhou, Z., Yuan, Q., Mash, D. C., & Goldman, D. (2017). Substance-specific and shared transcription and epigenetic changes in the human hippocampus chronically exposed to cocaine and alcohol. Proceedings of the National Academy of Sciences, 114(20), 5685–5690. View
Tapocik, J. D., Solomon, M., Flanagin, M., Meinhardt, M., Estelle, B., Schank, J., Schwandt, M., Sommer, W.H., Heilig, M. (2013). Coordinated dysregulation of mRNAs and microRNAs in the rat medial prefrontal cortex following a history of alcohol dependence. The Pharmacogenomics Journal. 13 (3): 286-296. View
Cadet, J. L., & Bisagno, V. (2012). Epigenetics of drug abuse: predisposition or response. The Pharmacogenomics Journal. 13 (10): 1149-1160. View
Joushi, S., Esmaeilpour, K., Masoumi-Ardakani, Y., Esmaeili-Mahani, S., Sheibani, V. (2021). Effects of short environmental enrichment on early-life adversity induced cognitive alternations in adolescent rats. Journal of Neuroscience Research. 99 (12): 3373-3391. View
Rusconi, F., Battaglioli, E., & Venturin, M. (2020). Psychiatric Disorders and 1ncRNAs: A Synaptic Match. International Journal of Molecular Sciences, 21(9), 1–19. View
Grest, C.V., Cedarbaum, J.A., Lee, J.O., Unger, J.B. (2021). Adverse childhood experiences and the substance use behaviors of Latinx youth. Drug and Alcohol Dependence. 227 (2021) View
17. Blackson, T.C., Tarter, R.E., Mezzich, A.C. (1996). Interaction Between Childhood Temperament and Parental Discipline Practices on Behavioral Adjustment in Preadolescent Sons of Substance Abuse and Normal Fathers. The American journal of drug and alcohol abuse. 22 (3); 335-348. View