Journal of Rehabilitation Practices and Research Volume 1 (2020), Article ID: JRPR-110
https://doi.org/10.33790/jrpr1100110Mini Review
The Effects of the Suboccipital Release Technique on the Autonomic Nervous System in Healthy Subjects: a Pilot Study
Rob Sillevis, PT, DPT, PhD*,OCS, FAAOMPT, Kaylee Fichthorn, DPT $ Eric Shamus, DPT, PhD, CSCS
Department of Physical Therapy, Florida Gulf Coast University, Marieb Hall 428, Fort Myers, Florida 33965, United States.
Corresponding Author Details: Rob Sillevis, PhD, DPT, OCS, FAAOMPT, MTC, Assistant Professor, Assistant Program Director Doctor of Physical Therapy, Florida Gulf Coast University, Marieb Hall 428, Fort Myers, Florida 33965, United States. E-mail: rsillevis@fgcu.edu
Received date: 29th July, 2020
Accepted date: 23th August, 2020
Published date: 25th August, 2020
Citation: Sillevis, R. (2020). The effects of the suboccipital release technique on the autonomic nervous system in healthy subjects: a pilot study. J Rehab Pract Res 1(2):110.
Copyright: ©2020, This is an open-access article distributed under the terms of the Creative Commons Attribution License 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Abstract
Introduction: Neck pain and dysfunction are often the results of structural imbalances between the thoracic and cervical spine, including the suboccipital region. Myodural bridges between the suboccipital musculature, cervical fascia, and central nervous system present an essential consideration for the suboccipital release technique (SRT). Previous studies demonstrated local and systemic effects following SRT. Systemic effects resulting from SRT are likely due to the somatic influence on the autonomic nervous system (ANS). Previous research has suggested this influence. No prior studies have investigated the direct effect of SRT on the ANS in real-time. The ANS can be indirectly measured by pupil diameter.
Purpose: To determine the immediate and short-term effect of the suboccipital release on the ANS system in a subgroup of healthy individuals.
Methods: Twenty-seven subjects were screened for red flags and factors which may bias the ANS. The subjects underwent baseline pupillometry measurement, administration of SRT, and subsequent pupillometry measurement. Subjects also completed a Global Rate of Change Scale (GROC) following testing.
Results: The mean pre-pupil diameter of the left eye did not differ significantly between Post1 and Post2 measures (F= 0.942, p=0.395). For the right pupil assessment, the non-parametric Friedman’s test of differences among repeated measures was conducted and rendered a Chi-square value of 1.407, which was non-significant, p=0.495. Five subjects reported feeling unchanged after the measurement phase (18.5%). Nine subjects reported a “1“ score of change (33.3%, seven subjects reported a “2” score of change (25.9%), and 6 subjects reported a “3” score of change (22.25%). The mean score was 1.5185 (SD=1.05 and a SE=0.202).
Conclusion: This study’s results demonstrate that the SRT did not have a significant immediate short-term systemic effect on the ANS. There was an overall perception of our subjects that something beneficial changed based on the GROC.
Introduction
Forward head posture is a common clinical presentation. It presents as a misalignment of the head on the trunk, causing the surrounding musculoskeletal system to compensate. The cervical spine posterior musculature becomes shortened and tightened while the anterior musculature becomes lengthened and weakened with stress on the intervertebral disc, ligaments, muscles, facet joints, dura, and nerve roots [1]. This imbalance results in neck dysfunction and pain for which individuals will seek healthcare [2,3]. Neck pain can arise from any structure in the neck and thoracic spine including the suboccipital region [4,5].
The motion of the suboccipital region is controlled by several groups of both smaller and larger muscle groups. Roijezon et al. [6] demonstrated that abnormal cervical functioning would change the proprioceptive awareness coming from this region. Proprioception is the information sent by mechanoreceptive neurons to the brain, informing it of the position of the neck, head, and any positional changes. There is an exceptionally high density of mechanoreceptors in the suboccipital musculature [6]. With cervical dysfunction, proprioception may be impeded by inflammation, abnormal muscle tone and pain. Fascial connections between the rectus capitis posterior minor, rectus capitis posterior major, and the obliquus capitis inferior muscles, and the dura have been identified previously [7,8]. The significance of this “myodural bridge” is that besides the neurogenic relationship between the head and neck, including proprioception, there is a direct anatomical connection between the suboccipital musculature, cervical fasciae, and the central nervous system. Furthermore, this relationship is supported by the fact that many clinicians experience the phenomenon of neural tension when treating patients with neck pain and headaches [9-11].
This anatomical connection between the suboccipital musculature and the central nervous system is essential when considering the direct effects of the Suboccipital Release technique (SRT). The Suboccipital Release technique is also termed nuchal line inhibition. Rodriguez-Huguet et al. [12] demonstrated that the SRT has a positive short-term effect on pain and pain pressure threshold in subjects with neck pain. Kim and Lee identified that SRT could result in an immediate change in muscle tone [1,13]. There is also evidence to support the more general effect of the SRT. Cho et al. [14] demonstrated an immediate change in length of the hamstrings after the suboccipital release technique was applied. Considering the lack of direct segmental relationship between these regions, it suggested an overall central effect. The only system with a relationship between the high cervical region and other regions of the body would be the autonomic nervous system (ANS). This relationship was supported by Metzler-Wilson et al. [15] who demonstrated that SRT can change the functioning of the ANS, especially when pain is induced.
Because the central nervous system functions as one single unit, both the somatic and ANS will be influenced by each other, and muscle tonicity and shorting in the suboccipital region could affect the ANS [16-18]. There are several interaction between the somatic and autonomic nervous system both in the periphery and centrally [19,20]. The consequences of these interaction are many and should be considered therapeutically. As an example, the regulation of muscle blood flow is controlled by the sympathetic nervous system [21-24]. Increased sympathetic activity will result in peripheral vasoconstriction and will reduce blood flow through the tissues [21]. If this state of increased sympathetic activity remains for a prolonged period of time ischemic changes in tissues can occur. This has been related with the development of myofascial trigger points. There will be a higher risk of tissue damage during the performance of routine activities [25].
The activity of the autonomic nervous system and its components can be assessed clinically [26,27]. The pupil of the eye is exclusively innervated by the ANS, and thus ANS activity can be measured indirectly by pupil monitoring [28-31]. The pupil dilator muscle is innervated by the sympathetic nervous system and the parasympathetic nervous system innervates the pupil constrictor muscle. The diameter of the pupil at any given time is the reflection of the real-time balance between the activity of the sympathetic system and parasympathetic activity [26,32,33]. Previous studies have used fully automated pupillometry to capture the pupil diameter in real-time [34-36]. No the previous studied have been reported of the direct effect of the SRT on the ANS. Therefore, this study aimed to determine the immediate and short-term effect of the suboccipital release on the autonomic nervous system in a subgroup of healthy individuals. It was hypothesized that a reduction in muscle tone should lead to an overall reduction of sympathetic activity. The secondary aim of this study was to investigate if the suboccipital release technique was perceived as creating a change using Global Rate of Change (GROC) and if this correlates to a change in nervous system activity.
Material and methods
A quasi-experimental pilot study design with a convenience sampling method was used for this study. Subjects were recruited from the staff and students of Florida Gulf Coast University in Fort Myers, Florida. Data collection took place between September 2019 and November 2019. This study received Institutional Review Board (IRB) approval from Florida Gulf Coast University.
Subjects
Because this was a pilot study, a power analysis was not performed. Twenty-seven subjected were recruited for this pilot study. All subjects were screened for eligibility criteria. To participate, all subjects had to be between the ages of 18 and 65, able to speak and read the English language fluently. The subjects were screened for by the treating physical therapist for any red flags and potential reasons for not undergoing the testing. Additional exclusion criteria included: evidence of central nervous system involvement, including hyperreflexia, nystagmus, loss of visual acuity, vertigo, an impaired sensation of the face, altered taste, and the presence of pathological reflexes.
Automated measures
The pupil diameter is a direct reflection of the functioning of the autonomic nervous system and can be measured directly in real time [29,31,33,37-39]. In this study, pupil responses were measured with the fully automated Vorteq® system (Micromedical Technologies, Inc) to record the pupil reaction. To control for any light affecting the pupil measures, the subjects wore goggles that covered both eyes to create a completely dark environment. In the dark, the parasympathetic nervous system’s activity is greatly reduced and, therefore, an increase in pupil diameter indicates a relatively unopposed activation of the sympathetic nervous system [32,39,40]. Infrared cameras are built into the goggles, allowing for direct measurement of the pupil diameter of both eyes simultaneously (Figure 1).
The use of fully automated pupillometry devices have been reviously reported [28,31,35,36,41-46]. The measurement error is minimal and pupil changes of less than 0.2 mm can be detected [44,46-48]. Intra-rater repeatability of automatic pupillometric devices is strong, with the coefficient of repeatability ranging from 0.6 to 1.4 mm [41,42]. The pupillometry’s sensitivity and reliability to evaluate the autonomic nervous system has been shown previously [28,49-51].
Pupillometry Testing
Study Protocol
All subjects provided written consent before participating in the study. All subjects completed a brief self-response survey, which collected data on the subject’s recent caffeine and over the counter Non-Steroidal Anti-Inflammatory (NSAID) medication use, and if the subject was experiencing pain at the time of data collection.
Measurement position
During the measurement phase, all subjects were in a comfortable supine position with the head resting on a pillow. This position was chosen because this is the position that is clinically most commonly used 13,14. It is the most comfortable position to deliver the SRT. Additionally, it allows the goggles to be worn comfortably and to guarantee that the infrared cameras would be able to capture the pupil during the measurement protocol without difficulty. All subjects remained in complete darkness, created by the goggles, for the duration of the intervention and measurement protocol. The subjects confirmed verbally that no light was entering the goggles. The darkness allowed for a constant maximum pupil diameter during the pupil measurement without the influence of light [52].
Measurement and Suboccipital Release protocol
After two minutes of accommodation to the dark environment, the pupils were measured continuously for a 60-second duration. Directly following the baseline measurement, the subject underwent the SRT. Both hands of the clinician cradled the subjects head. The dorsum of bilateral hands rested on the treatment table, and the fingertips of fingers 2 through 5 were placed in the suboccipital region bilaterally. After this, the MTP joints were flexed 90 degrees and the PIP and DIP joints were extended. This hand and finger position resulted in an elevation of the neck and head, with concurrent pressure generated in the suboccipital tissues due to gravitational force on the head. This position was maintained until the clinician perceived a noticeable reduction in tone in the suboccipital muscles. Directly following the SRT, a continuous pupil measurement of both eyes was performed for 60-seconds. After 3-minutes the third and final 60-second pupil measurement was taken, which completed the pupillometry measurement phase. The testing environment was temperaturecontrolled and remained the same for all subjects during this pilot study.
Global Rate of Change Scale
Immediately following pupillometry testing, subjects were asked to complete a Global Rate of Change (GROC) scale. The GROC was used to determine if they felt any change after the administration of the suboccipital release. Additionally, the subjects had the opportunity to record any additional comments regarding the suboccipital release or their participation in the trial.
Statistical analysis
Statistical analyses were performed using the SPSS, version 26.0, statistical software package. The data were analyzed for normal distribution using the Sharpo-Wilk test. All data were normally distributed for the left pupil, so the repeated measure ANOVA was used to analyze the data. The pre-pupil measurement and the post1 measurement for the right eye were not normally distributed so nonparametric Friedman’s ANOVA analysis was used to analyze the data.
Results
Baseline characteristics
A total of 27 subjects were assessed for eligibility and enrolled in the study. Twelve subjects out of 27 subjects were male (44.4%), while 15/27 were female (55.6%), and the mean subject age was 27.29, with a range of 21 to 60 years.
Systemic effect of the suboccipital release technique
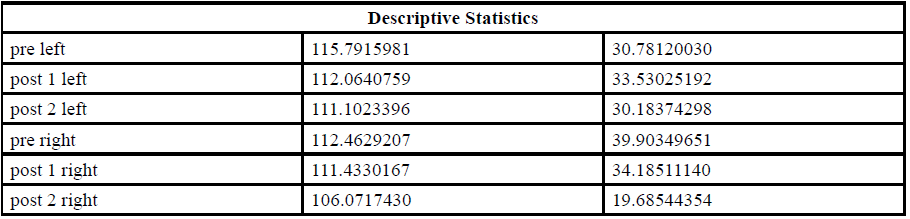
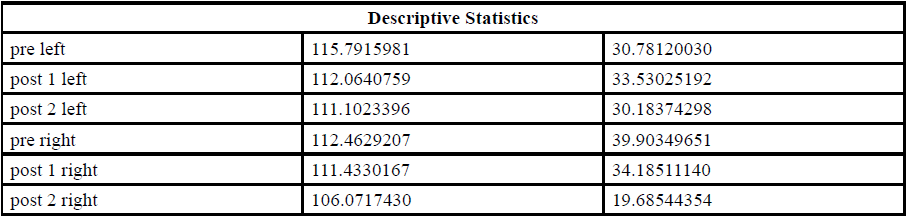
In order to determine the immediate effect of the SRT on the ANS, the pre-test pupil diameter was compared with the pupil measurement taken immediately after the SRT(Post1) and 4 minutes later (Post2). The assumptions for the use of parametric statistics were satisfied for the left eye. Thus, a repeated measure ANOVA test was performed to compare the pre- and post-SRT pupil diameter. The assumptions for the use of parametric statistics were not satisfied for the right eye. Thus, the Friendman’s ANOVA test was performed to compare the pre- and post-SRT pupil diameter. For the pupil diameter mean for each of the measures, see Table 1 for both eyes.
Left pupil:
The repeated measure ANOVA with a Greenhouse-Geisser correction showed that the mean pre-pupil diameter of the left eye did not differ significantly between Post1 and Post2 measures (F= 0.942, p=0.395). This test yielded an observed power of 0.203. (Table 2)
Right pupil
The non-parametric Friedman’s test of differences among repeated measures was conducted and rendered a Chi-square value of 1.407, which was non-significant at p=0.495.
Global Rating of Change Scale
Each subject completed a Global Rating of Change Scale using a 5-point Likert scale (-5 indicating “very much worse”, 0 indicating “no change”, and 5 indicating “Completely gone”). Five subjects reported feeling unchanged after the measurement phase (18.5%). Nine subjects reported a “1“ score of change (33.3%, seven subjects reported a “2” score of change (25.9%) and six subjects reported a “3” score of change (22.25%). The mean score was 1.5185 (SD=1.05 and a SE=0.202), supporting the hypothesis of an overall self-perceived benefit from the intervention.
Discussion
This study aimed to evaluate the immediate short-term effect of the suboccipital release technique in a subgroup of healthy subjects on the autonomic nervous system. Additionally, it set out to investigate the short-term self-perceived changes following the SRT. This study’s results demonstrate that the SRT did not have a significant immediate short-term systemic effect on the ANS.
It was previously demonstrated that the SRT would have a systemic effect [14]. It appears that these effects cannot be explained through peripheral and segmental relationships [14]. The most appropriate system that could achieve remote changes is the autonomic nervous system (ANS). This is supported by the findings of Metzler-Wilson et al. [15] who demonstrated that SRT can change the functioning of the ANS. We used automated pupillometry to measure the pupillary response to the manual application of pressure force in the suboccipital region. Previous research has validated pupillometry as a reliable method of assessing the autonomic nervous system without the presence of examiner bias [28,29,39,41,42,44,47,48,53,54].
To our knowledge, this pilot study is the first of its kind that has attempted to measure the immediate effect of the SRT on the overall activity of the autonomic nervous system. This study supports the findings of Rodriguez-Huguet et al. [12] that the SRT has a positive short-term effect on pain. Although we used asymptomatic individuals for this study, 81.5% of our subjects reported feeling better after the application of the SRT.
Based on the pupillary changes observed and the GROC results, it appears that there was an immediate effect of the SRT on the nervous system. However, it did not create a statistically significant measurable systemic change in autonomic nervous system functioning. The sympathetic nervous system innervates the pupil dilator muscle and the parasympathetic nervous system innervates the pupil constrictor muscle. The diameter of the pupil at any given time is a reflection of the real-time balance between the activity of the sympathetic system and parasympathetic activity [26,32,33]. Our results indicate a more constricted pupil in both eyes in each measure point post SRT. A constricted pupil implies a more dominant parasympathetic or decreased sympathetic activity. One reason for this non-significant finding could be the relatively small sample size. This pilot study results demonstrate a trend in which pupil diameter decreased in each post-intervention measurement. It could be possible that if there were additional measures passed the 4-minute post-intervention time frame a significant change in pupil diameter could be found. Additional research is needed to evaluate the longerterm effect of the SRT on the ANS.
The superior cervical vertebral column is a very complex anatomical region. There appears to be a clear anatomical relationship between muscular, ligamentous, soft tissues, and the dura mater in the high cervical region. Individuals with cervical related dysfunctions can present clinically with a variety of musculoskeletal myofascial syndromes in the upper quadrant and reduced active and passive movements [55] Cervical dysfunction often leads to the typical upper cross muscle syndrome with forward head posture [56]. In this position, the head is relatively forward, and this will lead to a posterior rotation of the occiput on the atlas and a relative backward bend of the atlas on the axis in the sagittal plane. This upper cervical spine position will result in forward flexion of the lower cervical vertebrae and increased lordosis in the mid to upper cervical region. In this position muscle adaptation will occur over time. The suboccipital muscles (rectus capitis posterior major, rectus capitis posterior minor, obliquus capitis superior, and obliquus capitis inferior), sternocleidomastoid, and scalenes are kept in the shortened position, which could lead to muscular tightness. In a study by Fernández de las Peñas et al. [57], the degree of forward head posture was positively correlated with the presence of suboccipital trigger points. Scali et al. [8] and Pontel et al. [7] suggest that increased tension of the suboccipital muscles could result in neural tension due to the presence of the myodural bridges. Kim and Lee identified that SRT could result in an immediate change in muscle tone [1,13]. The results of our study support this finding and thus supports the notion that the Suboccipital Release Technique can be used as a clinical approach to directly reduce muscle tone in the suboccipital region. This concurs with the findings of Jiang et al. [58], who demonstrated that the use of SRT was beneficial in a treatment approach for subjects with tension-type headaches.
The design of this pilot study has several limitations. First, it had a small subject sample, which limits the generalizability of the findings. Additionally, gender representation was not equal, as the subject sample included 12 males and 15 females. However, it is unlikely to have negatively affected this study’s outcome since it was previously demonstrated that gender difference had no impact on the pupil response to stimuli [59]. Although asymptomatic individuals were during this study it is possible that the initial application of the force through the fingers of the clinician to the suboccipital region was experienced as uncomfortable by the subjects and thus affected the pupil measurements. We did not control for this potential issue. The subjects for this pilot study were selected through convenience sampling, and the results of this pilot study could be different in subjects with painfull conditions, which should be considered in follow-up studies. Based on the fact that pain has a direct stimulating effect on the sympathetic nervous system follow up studies should evaluate if a “pain subject group” would have a different response than the subjects in this pilot study on the SRT. Additionally, the inclusion criteria did not control for substances that could affect the autonomic nervous system such as caffeinated beverages and or OTC medication. This was intentionally not controlled, as this is typically not controlled during patient care either. However, we have to consider that this might have affected the study results. Furthermore,there was no control of the age of the subjects and it is possible this could have affected the pupillary response.
Conclusion
This pilot study’s results demonstrate that in a subgroup of healthy individuals the suboccipital release technique results in decreased muscle tone in the suboccipital region and a decreased diameter of the pupils of both eyes. A pupil constriction would imply a reduction in the activity of the sympathetic nervous system; however, the results were not statistically significant. There was an overall perception of our subjects that something beneficial changed based on the Global Rating of Change Scale.
Conflicts of interest/Competing interests:
Authors report no conflict or competing interest.
References
Kim, S. J., & Lee, J. H. (2018). Effects of sternocleidomastoid muscle and suboccipital muscle soft tissue release on muscle hardness and pressure pain of the sternocleidomastoid muscle and upper trapezius muscle in smartphone users with latent trigger points. Medicine (Baltimore), 97(36), e12133. doi:10.1097/MD.0000000000012133View
Strine, T., & Hootman, J. (2007). US National Prevelance and Correlations of Low Back and Neck Pain Among Adults. Arthritis & Rheumatism (Arhtritis Care & Research), 57(4), 656-665.View
Taylor, H., & Murphy, B. (2008). Altered Sensorimotor Integration With Cervical Spine Manipulation. Journal of Manipulative and Physiological Therapeutics, 31(2), 116-125.View
Bogduk, N. (1994). Innervation and Pain patterns of the Cervical Spine. In Clinics in Physical Therapy: Physical Therapy of the Cervical and Thoracic Spine (pp. 65-76). New York: Churchill Livingstone.
Krauss, J., Creighton, D., Ely, J., & Podlewska-Ely, J. (2008). The Immediate Effects of Upper Thoracic Translatoric Spinal Manipulation on Cervical Pain and Range of Motion: A Randomized Clinical Trial Journal of Manual and Manipulative Therapy, 16(2), 93-99.View
Roijezon, U., Clark, N. C., & Treleaven, J. (2015). Proprioception in musculoskeletal rehabilitation. Part 1: Basic science and principles of assessment and clinical interventions. Man Ther, 20(3), 368-377. doi:10.1016/j.math.2015.01.008View
Pontell, M. E., Scali, F., Marshall, E., & Enix, D. (2013). The obliquus capitis inferior myodural bridge. Clin Anat, 26(4), 450-454. doi:10.1002/ca.22134View
Scali, F., Marsili, E. S., & Pontell, M. E. (2011). Anatomical connection between the rectus capitis posterior major and the dura mater. Spine (Phila Pa 1976), 36(25), E1612-1614. doi:10.1097/BRS.0b013e31821129dfView
Caamano-Barrios, L. H., Galan-Del-Rio, F., Fernandez-de-Las- Penas, C., Cleland, J. A., Plaza-Manzano, G., & Ortega-Santiago, R. (2019). Evaluation of neurodynamic responses in women with frequent episodic tension type headache. Musculoskelet Sci Pract, 44, 102063. doi:10.1016/j.msksp.2019.102063View
Szikszay, T. M., Luedtke, K., & Harry von, P. (2018). Increased mechanosensivity of the greater occipital nerve in subjects with side-dominant head and neck pain - a diagnostic case-control study. J Man Manip Ther, 26(4), 237-248. doi:10.1080/106698 17.2018.1480912View
von Piekartz, H. J., Schouten, S., & Aufdemkampe, G. (2007). Neurodynamic responses in children with migraine or cervicogenic headache versus a control group. A comparative study. Man Ther, 12(2), 153-160. doi:10.1016/j. math.2006.06.004View
Rodriguez-Huguet, M., Gil-Salu, J. L., Rodriguez-Huguet, P., Cabrera-Afonso, J. R., & Lomas-Vega, R. (2018). Effects of Myofascial Release on Pressure Pain Thresholds in Patients With Neck Pain: A Single-Blind Randomized Controlled Trial. Am J Phys Med Rehabil, 97(1), 16-22. doi:10.1097/ PHM.0000000000000790View
Kim, B. B., Lee, J. H., Jeong, H. J., & Cynn, H. S. (2016). Effects of suboccipital release with craniocervical flexion exercise on craniocervical alignment and extrinsic cervical muscle activity in subjects with forward head posture. J Electromyogr Kinesiol, 30, 31-37. doi:10.1016/j.jelekin.2016.05.007View
Cho, S. H., Kim, S. H., & Park, D. J. (2015). The comparison of the immediate effects of application of the suboccipital muscle inhibition and self-myofascial release techniques in the suboccipital region on short hamstring. J Phys Ther Sci, 27(1), 195-197. doi:10.1589/jpts.27.195View
Metzler-Wilson, K., Vrable, A., Schaub, A., Schmale, T. K., Rodimel, B. V., Krause, B. A., & Wilson, T. E. (2020). Effect of Suboccipital Release on Pain Perception and Autonomic Reflex Responses to Ischemic and Cold Pain. Pain Med. doi:10.1093/ pm/pnaa051View
Barman, S., & Wurster, R. (1978). Interaction of descending spinal sympathetic pathways and afferent nerves. American Journal of Physiology, 234(3), H223-H229.View
Benarroch, E. (2006). Pain-autonomic interactions. Neurological sciences, 27(Suppl 2), S130-S133.View
Van Cranenburgh, B. (1989). Inleiding in the toegepaste neurowetenschappen (Vol. 1). Lochem: Uitgeversmaatchappij de Tijdstroom.View
Benarroch, E. (2001). Pain-autonomic interactions: a selective review. Clinical Autonomic Research, 11, 343-349.View
Zusman, M. (2002). Forebrain-mediated sensitization of central pain pathways: 'non-specific' pain and a new image for MT. Manual Therapy, 7(2), 80-88.View
Rowell, L. (1997). Neural Control of Muscle Blood Flow: Importance During Dynamic Exercise. Clinical and Experimental Pharmacology and Physiology, 24, 117-125.View
Segal, S. (1994). Cell-to-cell communication coordinates blood flow control. Hypertension, 23, 1113-1120.View
Segal, S. (2005). Regulation of Blood Flow in the Microcirculation. Microcirculation, 12, 33-45.View
Thomas, G., & Segal, S. (2004). Neural control of muscle blood flow during exercise. Journal of Applied Physiology, 97, 731- 738.View
Maguire, A., Craig, M., Craighead, A., Chan, A., Cusumano, J., Hing, S., . . . Donaghue, K. (2007). Autonomic Nerve Testing Predicts the Development of Complications. Diabetes Care, 30(1), 77-82.View
Butler, D. (2000). The Sensitive Nervous System. Adelaide, Australia: Noigroup Publications.View
Menck, J., Requejo, S., & Kulig, K. (2000). Thoracic Spine Dysfunction in Upper Extremity Complex Regional Pain Syndrome Type I. Journal of Orthopaedic & Sports Physical Therapy, 30(7), 401-409.View
Bertinotti, L., Pietrini, U., Del Rosso, A., Casale, R., Colangelo, N., Zoppi, M., & Matucci-Cerinic, M. (2002). The Use of Pupillometry in Joint and Connective Tissue Diseases. New York Academy of Sciences, 966, 446-455View
Bitsios, P., Prettyman, R., & Szabadi, E. (1996). Changes in Autonomic Function with Age: A Study of Pupillary Kinetics in Healthy Young and Old People. Age and Ageing, 25, 432-438.View
Capao Filipe, J., Falcao-Reis, F., Castro-Correia, J., & Barros, H. (2003). Assessment of autonomic function in high level athletes by pupillometry. Autonomic Neuroscience: Basic and Clinical, 104, 66-72.View
Fotiou, F., Fountoulakis, K., Goulas, A., Alexopoulos, L., & Palikaras, A. (2000). Automated standardized pupillometry with optical method for purposes of clinical practice and research. clinical physiology, 20(5), 336-347.View
Bernards, J., & Bouman, L. (1988). Fysiologie van de mens. Utrecht: Bohn, Scheltema & Holkema.
Gibbons, P., Gosling, C., & Holmes, M. (2000). Short-Term Effects of Cervical Manipulation on Edge Light Pupil Cycle Time: A Pilot Study. Journal of Manipulative and Physiological Therapeutics, 23(7), 465-469.View
Sillevis, R., & Cleland, J. (2011). Immediate effects of the audible pop from a thoracic spine thrust manipulation on the autonomic nervous system and pain: a secondary analysis of a randomized clinical trial. J Manipulative Physiol Ther, 34(1), 37-45. doi:S0161-4754(10)00330-1 [pii] 10.1016/j. jmpt.2010.11.007View
Sillevis, R., Cleland, J., Hellman, M., & Beekhuizen, K. (2010). Immediate effects of a thoracic spine thrust manipulation on the autonomic nervous system: a randomized clinical trial. J Man Manip Ther, 18(4), 181-190. doi:10.1179/10669811 0X12804993427126View
Sillevis, R., Van Duijn, J., Shamus, E., & Hard, M. (2019). Time effect for in-situ dry needling on the autonomic nervous system, a pilot study. Physiother Theory Pract, 1-9. doi:10.1080/09593 985.2019.1644691View
Dutsch, M., Hilz, M., Raunhut, U., Solomon, J., Neundorfer, B., & Axelrod, F. (2002). Sympathetic and parasympathetic pupillary dysfunction in familial dysautonomia. Journal of Neurological Sciences, 195, 77-83.View
Harle, D., Wolffsohn, J., & Evans, B. (2005). The pupillary light reflex in migraine. Ophthal. Physiol. Opt, 25(3), 240-245.View
Pfeifer, M., Cook, D., Brodsky, J., Tice, D., Parrish, D., Reenan, A., . . . Porte, D. (1982). Quantitative Evaluation of Sympathetic and Parasympathetic Control of Iris Function. Diabetes Care Diabetes Care, 5(5), 518-528.View
Bakes, A., Bradshaw, M., & Szabadi, E. (1990). Attentuation of the pupillary light reflex in anxious patients. Br. J. clin. Pharmac., 30, 377-381.View
Boxer Walcher, B., & Krueger, R. (1999). Agreement and Repeatability of Infrared Pupillometry and the Comparison Method. Ophthalmology, 106(2), 319-323.View
Boxer Walcher, B., & Krueger, R. (2000). Agreement and repeatability of pupillometry using videokeratography and infrared devices. Journal of Cataract Refract Surg, 26(January), 35-40.View
Levy, D., Rowley, D., & Abraham, R. (1992). Portable infrared pupilloemtry using Pupilscan: relation to somatic and autonomic nerve function in diabetes mellitus. Clin. Auton. Res., 2, 335- 341.View
Meeker, M., Du, R., Bachetti, C., Larson, M., Holland, M., & Manley, G. (2005). Pupil Examination: Validity and Clinical Utility of an Automated Pupillometer. Journal of Neuroscience Nursing, 37(1), 34-40.View
Piha, S., & Halonen, J.-P. (1994). Infrared pupillometry in the assessment of autonomic function. Diabetes Research and Clinical Practice, 26, 61-66.View
Pop, M., Payette, Y., & Santoriello, E. (2002). Comparison of the pupil card and pupillometer in measuring pupil size. Journal of Cataract Refract Surg, 28(February), 281-288.View
Taylor, W., Chen, J., Meltzer, H., Gennarelli, T., Kelbch, C., Knowlton, S., . . . Marshall, L. (2003). Quantitative pupillometry, a new technology: normative data and preliminary observations in patients with acute head injury. Journal of Neurosurg., 98(January), 205-213.View
Twa, M., Bailey, M., Hayes, J., Bullimore, M., & McOptom. (2004). Estimation of pupil size by digital photography. Journal of Cataract Refract Surg, 30(February), 382-289.View
Neil, H., & Smith, S. (1989). A simple clinical test of pupillary autonomic function, Correlation with cardiac autonomic function tests in diabetes. Neuro-ophtalmology, 9(4), 237-242.View
Giakoumaki, S., Hourdaki, E., Grinakis, V., Theou, K., & Bitsios, P. (2005). Effects of peripheral sympatehtic blockade with dapiprazole on the fear-inhibited light reflex. Journal of Psychopharmacology, 19(2), 139-148.View
Miciele, G., Tassorelli, C., Martignoni, E., Marcheselli, S., Rossi, F., & Nappi, G. (1995). Further characterization of autonomic involvement in multiple system atrophy: a pupillometric study. Functional Neurology, 10(6), 273-280.View
Rickmann, A., Waizel, M., Kazerounian, S., Szurman, P., Wilhelm, H., & Boden, K. T. (2017). Digital Pupillometry in Normal Subjects. Neuroophthalmology, 41(1), 12-18. doi:10.1 080/01658107.2016.1226345View
Boev, A., Fountas, K., Karampelas, I., Boev, C., Machinis, T., Feltes, C., . . . Troup, C. (2005). Quantitative pupillometry: normative data in healthy pediatric volunteers. Journal of Neurosurg., 103(December).View
Merritt, S., Keegan, A., & Mercer, P. (1994). Artifact Management in pupillometry. Nursing Research, 43(1), 56-59.View
Borghouts, J., Koes, B., & Bouter, L. (1998). The clinical course and prognostic factors of non-specific neck pain: a systematic review. Pain, 77, 1-13.View
Cantu, R., & Grodin, A. (2001). Myofascial Manipulation, Theory and Clinical application second edition. Gaithersburg, Maryland: Aspen publications.View
Fernandez de las Penas, C., Cuadrado, M. L., Gerwin, R. D., & Pareja, J. A. (2005). Referred pain from the trochlear region in tension-type headache: a myofascial trigger point from the superior oblique muscle. Headache, 45(6), 731-737. doi:HED05140 [pii]10.1111/j.1526-4610.2005.05140.xView
Jiang, W., Li, Z., Wei, N., Chang, W., Chen, W., & Sui, H. J. (2019). Effectiveness of physical therapy on the suboccipital area of patients with tension-type headache: A meta-analysis of randomized controlled trials. Medicine (Baltimore), 98(19), e15487. doi:10.1097/MD.0000000000015487View
Ellermeier, W., & Westphal, W. (1995). Gender differences in pain ratings and pupil reactions to painful pressure stimuli. Pain, 61, 435-439.View