Journal of Rehabilitation Practices and Research Volume 5 (2024), Article ID: JRPR-150
https://doi.org/10.33790/jrpr1100150Research Article
REPAIR-EDS: Rehabilitation Enhanced by Partial Arterial Inflow Restriction (REPAIR) in Eheler-Danlos Syndrome (EDS) Patients
Justin Z. Laferrier1*, PT, PhD, NCS, OCS, SCS, ATP, CSCS, Taylor Mederios2, DPT, Kenneth Shin2, DPT, Sebastian Valdes2, DPT, and Mariusz Furmanek2, DPT, PhD
1Department of Physical Therapy, Johnson and Wales University, 8 Abbott Place, Providence RI 02903,United States.
2Department of Physical Therapy, University of Rhode Island, 25 West Independence Way, Suite J, Kingston RI, 02881,United States.
Corresponding Author Details: Justin Z. Laferrier, PT, PhD, NCS, OCS, SCS, ATP, CSCS, Associate Professor, Department of Physical Therapy, Johnson and Wales University, 8 Abbott Place, Providence RI 02903,United States.
Received date: 14th July, 2024
Accepted date: 27th August, 2024
Published date: 29th August, 2024
Citation: Laferrier, J. Z., Mederios, T., Shin, K., Valdes, S., & Furmanek, M., (2024). REPAIR-EDS: Rehabilitation Enhanced by Partial Arterial Inflow Restriction (REPAIR) in Eheler-Danlos Syndrome (EDS) Patients. J Rehab Pract Res, 5(1):150.
Copyright:©2024, This is an open-access article distributed under the terms of the Creative Commons Attribution License 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Introduction
The purpose of the current study was to test the effectiveness of combining blood flow restriction with standard physical therapy (PT BFR) exercises when compared to physical therapy exercises alone in a population of individuals living with Ehlers-Danlos Syndrome (EDS). EDS encompasses a complex spectrum of heritable connective tissue disorders characterized by genetic mutations affecting collagen synthesis and processing [1]. These mutations are inherited in varying patterns including autosomal dominant, recessive, and de novo mutations and lead to joint hypermobility, dermal dysplasia, and tissue fragility among other symptoms [2,3]. The most recent EDS classifications comprise at least 14 subtypes [4], each presenting unique clinical features and severity levels, affecting an estimated prevalence of 1 in 600 to 1 in 900 individuals depending on subtype [4-6]. Depending on the subtype, the syndrome can significantly impact functional capacity, quality of life, and longevity due to chronic pain, neuromusculoskeletal deficits, and other associated complications [7-12].
Historically, traditional therapeutic approaches for EDS, which include pain management, surgery, and rehabilitation aimed at correcting deficits and improving strength and joint stability, often fall short of achieving long-term efficacy [13]. This inadequacy necessitates exploration of innovative interventions capable of addressing the underlying muscle weakness and functional impairments characteristic of the syndrome.
Background
Since EDS affects collagen formation and function, it can potentially affect every organ system, leading to a myriad of dysfunctions resulting in significant morbidity and mortality [14-20]. Muscle weakness is a pervasive issue in musculoskeletal conditions worldwide, including EDS, leading to diminished functional abilities and reduced quality of life [21-24]. Resistance training, traditionally prescribed at heavy loads to promote muscle hypertrophy and strength gains, poses challenges for individuals with EDS due to their susceptibility to joint injuries and tissue fragility. Current guidelines from the American College of Sports Medicine advocate for load percentages ranging from 60-100% of one's maximum capacity [18], which may not be feasible or safe for EDS patients.
In response to these challenges, Blood Flow Restriction (BFR) training has emerged as a promising alternative intervention to conventional strength/ resistance exercises. BFR involves applying a specialized tourniquet system to partially restrict arterial inflow and venous outflow during low-intensity resistance exercises. This technique allows for significant muscular adaptations, including increased muscle protein synthesis and muscle hypertrophy, even at remarkably low loads (e.g., 20-50% of one's maximum capacity) [25-28]. The safety and efficacy of BFR have been well-documented across various populations, demonstrating its potential to enhance strength and mitigate muscle atrophy without subjecting joints to excessive stress [29-34].
Recent meta-analyses have underscored BFR's effectiveness in inducing muscle mass gains comparable to conventional high-load resistance training [35]. Mechanistically, BFR augments muscle fiber activation and promotes myonuclei proliferation, effects typically associated with higher-intensity training regimens [36-41]. Moreover, BFR has gained recognition across the lifespan in clinical and non-clinical settings, ranging from professional sports teams to rehabilitation centers, illustrating its versatility and acceptance as a therapeutic modality [42-46].
Innovation
The present study introduces the Rehabilitation Enhanced by Partial Arterial Inflow Restriction (REPAIR) intervention, combining conventional PT exercises with BFR training, as a novel approach for individuals with EDS. By integrating the benefits of low-load resistance training with the physiological effects of BFR, this research aims to optimize muscle hypertrophy and strength gains while mitigating the risk of joint injury and exacerbating symptoms. This pioneering effort seeks to stimulate future research dedicated to establishing evidence-based guidelines for the safe and effective implementation of BFR in clinical EDS rehabilitation, offering a promising alternative to conventional therapies.
The innovative potential of this study lies in its application of BFR to a population historically underserved by traditional exercise paradigms. By enabling EDS patients to engage in low-intensity exercises that effectively promote muscle strength and function without compromising joint integrity, REPAIR-EDS holds promise for improving long-term outcomes and enhancing quality of life. This research represents a critical step toward addressing the unmet needs of individuals with EDS, offering hope for more effective and sustainable management strategies.
The primary aims of the study were: 1. To compare the effect of PT-BFR versus standard PT care on self-reported and standardized outcome measures 2. To compare the effect of PT-BFR versus standard PT care on knee extensor and knee flexor strength. And 3. To compare the effect of PT-BFR versus standard PT care on muscle volume. All measures were taken at baseline, 3, 6 and 9-months during treatment and at the 12th month following treatment to determine whether the intervention sustained lasting effects.
Methods
Study Design
This study was a randomized controlled trial. Participants who meet the inclusion criteria were randomly assigned to one of two groups: a standard physical therapy (PT) exercises group or a group receiving standard PT exercises with the addition of Blood-Flow Restriction (PT-BFR) using a tourniquet system. Approval was obtained from University of Rhode Island Institutional Review Board #1821184. The experimental consort flow chart is shown in figure1.
Participants
Participants were recruited from various healthcare facilities in Rhode Island, Massachusetts, Connecticut, Vermont, and New Hampshire. Prior to enrollment, potential participants underwent an initial screening assessment conducted by a research team member to discuss the study objectives and review the consent form before signing. Inclusion and exclusion criteria were assessed during this meeting, and individuals meeting any exclusion criteria were not enrolled in the study. We enrolled 21 participants reflecting the two most common subtypes of EDS (17 participants had a diagnosis of Hypermobile EDS (hEDS) and 4 participants had a diagnosis of Classical EDS (cEDS)). 18 participants completed the study (15 participants with Hypermobile EDS (hEDS) and 3 participants with Classical EDS (cEDS).
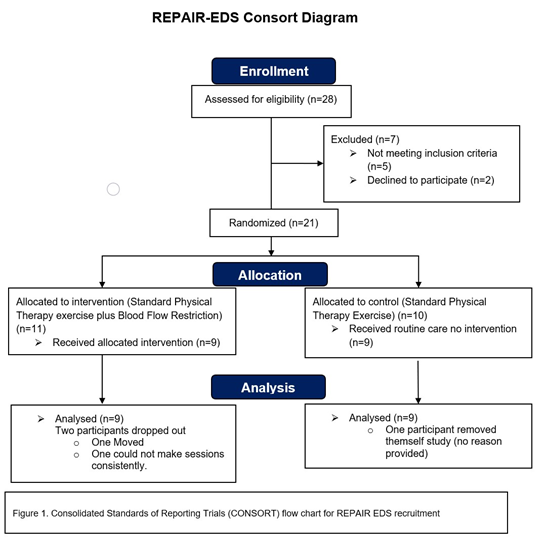
Figure 1. Consolidated Standards of Reporting Triais (CONSORT) flow chart for REPAIR EDS recruitment
Inclusion Criteria:
Participants were required to be between 18 and 65 years of age and have a diagnosis of EDS confirmed by a physician.
Exclusion Criteria:
Individuals were excluded if they presented with conditions such as additional injuries or medical conditions compromising lower extremity weight-bearing ability or engagement in physical therapy, history of vascular reconstruction, skin graft, anxiety disorders, diagnosis of vascular EDS, history of peripheral artery disease and/ or peripheral vascular disease, documented psychiatric disorders, inability to communicate in English or Spanish, known pregnancy, inability to obtain clearance for physical activity, and significant difficulties in maintaining follow-up appointments.
Randomization
Randomization procedures were rigorously implemented to minimize bias and ensure the validity of the findings in the REPAIR EDS study. Randomization was conducted during the initial assessment phase, where participants were assigned to either the standard physical therapy (PT) group or the physical therapy with blood-flow-restriction (PT-BFR) group. This random assignment was performed using simple random sampling without replacement.
Procedures
During the initial assessment, the potential participant met with a research team member where they discussed the study and reviewed the consent form. Exclusion criteria were reviewed and if the individual presented with any of the criteria they were not enrolled in the study. Once the potential participant reviewed the consent form and asked any questions, they were asked to sign the consent form and proceed with the initial baseline assessment. The initial assessment consisted of gathering demographic information, self-report and standardized outcome measures, and measures of muscle strength and volume as previously listed in the specific aims. Time commitment of the initial assessment was not standardized and was based on the participant's overall condition, although attempts were made to conduct the initial assessment within one-two hours. Subsequent assessments occurred at 3, 6, 9, and 12 months after the start of treatment, each lasting approximately 1 hour. These assessments included the same measures administered during the initial assessment. Any complications that occurred due to treatment or events outside of treatment were addressed at the time of incident and assessed during patient examination. During the initial assessment, participants were randomly assigned to one of two groups, one only receiving standard PT exercises (PT) and the other receiving the same treatment with the addition of Blood-Flow-Restriction (PT BFR). Both treatment groups participated in 9 months of intervention 2-3 times per week. Both groups completed the same exercises, but the sets, reps, and intervals were adjusted depending on participant progression. Once 9 months of treatment had been completed the participant received a standard home exercise program.
Intervention
The PT treatment group (control group) received conventional physical therapy exercises consisting of standardized knee extensions and straight leg raises 2-3 times per week for a duration of 9 months. The sequencing of the exercises was standardized so that supine single leg straight leg raises were performed first with the left lower limb then the right lower limb, then the participant would move to the seated position and perform single leg knee extensions first with the left lower limb then the right lower limb. Each exercise consisted of 4 sets: the first set comprising 30 repetitions and subsequent sets comprising 15 repetitions each. There was a 30-second rest period between each set. Each repetition was performed at a tempo of 4 seconds total (2 seconds concentric and 2 seconds eccentric contractions).
Participants in the PT-BFR treatment group (experimental group) received the same standardized PT exercises described above except leg exercises (i.e. leg extensions and straight leg raises) were completed wearing an FDA approved tourniquet system. In this case A Delfi PTSII tourniquet system (Delfi Medical Vancouver, BC) was used [47]. This tourniquet system is similar to a blood pressure cuff that is used to decrease the arterial inflow to stimulate the benefits of BFR. This system has been extensively used in BFR research and training and has been found to be safe and reliable to the degree that it is the only system used by several BFR companies. The tourniquet was applied proximally on the limb performing the exercise at a pressure determined to occlude flow at 80% of arterial occlusion value. This pressure was maintained throughout the exercise session. The total tourniquet time, including rest periods, did not exceed 5 minutes and 30 seconds per exercise session. The Delfi PTSII tourniquet system includes a built-in deflation feature that activates at the end of the 5 minutes and 30 seconds to release the tourniquet pressure.
In the case the individual could not perform all sets and repetitions the number of repetitions performed was recorded. Adjustments were made based on participant progress and included increasing external resistance by using ankle weights in .5 lb. increments. After 9 months, treatment was discontinued, and participants received a standardized home exercise program.
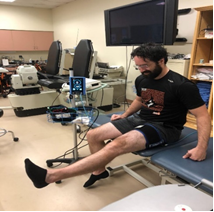
Figure 2. Knee extension exercises with Blood-Flow-Restriction implemented by the Delfi PTSII tourniquet system.
Outcome Measures
Outcome measures included changes in subjective pain, muscle strength, volume, and self-report measures.
1. Brief Pain Inventory (BPI): The BPI assessed pain severity, its interference with daily functioning, location, pain medication usage, and pain relief efficacy over the past week, providing a valid, reliable, and responsive measure of pain [48].
2. Bilateral Knee Flexion and Extension Strength: Was evaluated using hand-held dynamometry, providing quantitative and objective measures of muscle strength. Measurements were taken using a wireless digital microFET2 hand-held dynamometer (Hoggan Scientific LLC, Drapper, UT, USA). Measurements using the belt-stabilized hand-held dynamometer are considered valid and highly reliable. All research personnel were trained in a standardized protocol when taking measurements to limit human error [49].
3. Ultrasonography: Cross-sectional areas of the vastus lateralis and lateral head of the gastrocnemius were measured using ultrasonography. Ultrasonography has consistently been reported as a valid tool for skeletal muscle size quantification. vastus lateralis measurements were taken as a direct reflection of muscles benefiting from the exercises performed. Gastrocnemius measurements were taken to investigate potential distal benefits. All ultrasound measurements were performed by the same researcher to enhance reliability [50].
4. Patient Health Questionnaire - 9 (PHQ-9): A self-report questionnaire assessing the presence and severity of depressive symptoms over the past two weeks, with a suggestive cutoff score for depression set at greater than 11 points. The PHQ 9 is a valid and reliable instrument considered comparable to longer clinician-administered instruments in a range of settings, countries, and populations [51].
5. World Health Organization Quality of Life (WHOQOL BREF): Quality of life (QOL) was assessed by the WHOQOL BREF. The WHOQOL-BREF is a valid and reliable 26 item self-report questionnaire developed by the World Health Organization. Twenty-four items constitute four subdomains: physical health, psychological health, social relationships, and environment, whereas the other two items measure overall QOL and general health. The domain scores denote an individual’s perception of quality of life in each particular domain [52].
This study was conducted in accordance with relevant guidelines and regulations, and all participants provided informed consent prior to participation.
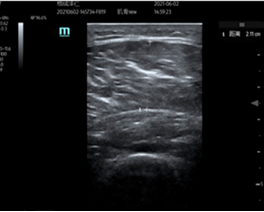
Figure 3. Example of ultrasound measurement of vastus lateralis. The “+” markers indicate the start and end points of the range of measurement.
Statistical Analysis
To analyze muscle strength and volume 2×5 repeated measures ANOVAs with a between subject variable identified as Group (Experimental=PT-BFR vs. Control=PT) and a within subject variable identified as Time (Baseline, 3-, 6-, 9-, and 12-months). A 2×5 repeated measure ANOVA was used to assess the self-reported outcome measures PHQ-9 and WHOQOL-Bref scores for domains 1-4. Significant effects were explored using pairwise contrasts with Bonferroni corrections. Statistical significance was set as at p <.05.
To verify the normality of data distribution, the Shapiro-Wilk test was employed, confirming that all variables met this assumption. The Mauchly's sphericity test assessed the sphericity assumption for repeated measures ANOVAs, and Greenhouse-Geisser corrections were applied to factors violating this assumption. The Levene's test ensured homogeneity of variance among the sample data, with all data satisfying this criterion. A threshold for statistical significance was established at p<0.05. All statistical analyses were conducted using Statistica (version 13, Dell Inc.). Results are presented as estimated marginal means. A power calculation was not performed prior due to this being a proof-of-concept pilot study.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
1. Brief Pain Inventory (BPI):
The BPI showed a significant improvement over time (F(4, 32)=5.78, p=.030, η2 =.67), regardless of the group. Interaction between group was also significant (F(4, 32)=15.73, p=.001, η2 =.67) reporting a significant improvement of the PT-BFR vs the PT group.
2. Bilateral Knee Flexion and Extension Strength
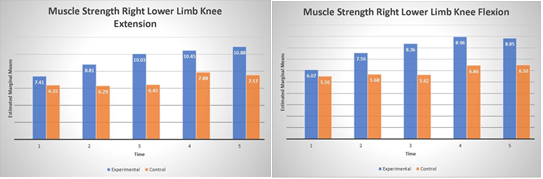
The analysis revealed a significant main effect for left knee flexion (F(1,8)=5.77, p=.004, η2 =.42) and significant main effect of left knee extension (F(1,8)=5.51, p=.047, η2 =.41) indicating that PT BRF group improved their strength on average by 2.7 kg in flexion and 3.6 kg in extension (see Fig. 4) compared to the PT group. Moreover, the main factor time was also significant for knee flexion (F(4,32)=11.30, p=.001,η2 =.58) and knee extension (F(4,32)=12.02, p=.001, η2 = .61). Interestingly, post hoc analysis revealed that the improvement is significant after 6 months of training comparing to baseline (all p’s<0.02). Additionally, for left knee flexion the significant interaction between time and group was observed (F(4, 32)=7.11 p=.001, η2 = .47). This effect indicates that after 9 months of BFR training there is tendency to reduce left knee flexion strength.
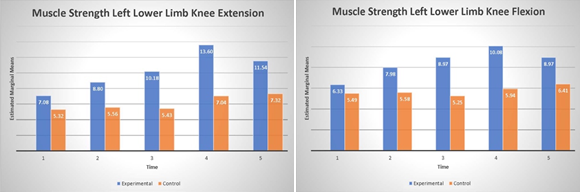
Figure 4. Muscle strength of left lower limb knee extension (panel A) and flexion (panel B) over time.
Analysis of the right lower limb showed no significant effect in both knee flexion or extension (p’s>.05). The lack of the effect may be explained that larger variability (larger std) observed in lower limb (see Fig. 4). The main factor of time revealed to be significant in both knee flexion (F(4, 32)=10.44, p=.001, η2 =.56) and extension (F(4, 32)=4.42, p=.005, η2 =.35) indicating that both groups significantly improve knee flexion after 6 months and extension after 9 months of training. Additionally right knee flexion showed significant interaction (F(4, 32) =2.96, p=.003, η2 =.27) between group and time indicating again that BFR group slightly decrease knee flexion strength comparing to the PT group.
3. Ultrasonography
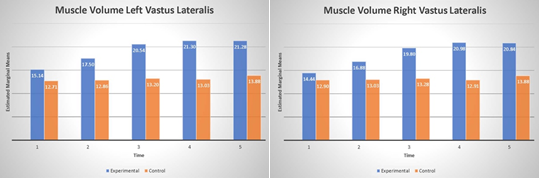
The analysis of ultrasound measurements revealed a significant main effect of group for both right (F(1, 8)=10.37, p=.01, η2 =.56) and left (F(1, 8) =10.87, p=.01, η2 =.58) vastus lateralis as well as main effect of time; right (F(4, 32) =14.1, p=.001, η2 =.64), left (F(4, 32) =13.52, p=.001, η2 =.63), and significant interactions between group and time for right (F(4, 32) =5.78, p=.001, η2 =.42), and left (F(4, 32) =4.21 p=.007, η2 =.34), see Fig. 5. This data indicates that volume on average increased 5.3 mm and 6 mm in the PT-BFR group for right and left extremity respectively. The significant main effect of time shows that improvement is seen after 6 months of training in both extremities (all p’s<.001). Significant interactions revealed that while the control- PT group improve the results after 9 months of training the PT-BFR group has the tendency to slightly reduce the volume of the quads.
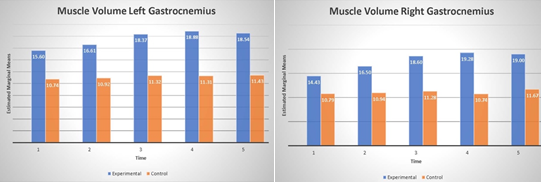
Very similar results were observed when ultrasound measurements of gastrocnemius is taken into account. The analysis showed the significant main effect of group for both right (F(1, 8) =23.12, p=.001, η2 =.74) and left (F(1, 8) =24.47, p=.001, η2 =.75) as well as main effect of time; right (F(4, 32) =16.5, p=.001, η2 =.67), left (F(4, 32) =10.38, p=.001, η2 =.56), and significant interactions between group and time for only right (F(4, 32) =6.62, p=.001, η2 =.45), see Fig. 6. The data indicates that calf volume on average increase 6.48 mm and 6.46 mm in the BFR group for right and left extremity respectively. The significant main effect of time shows that improvement is seen after 6 months of training in both extremities (all p’s<.001).
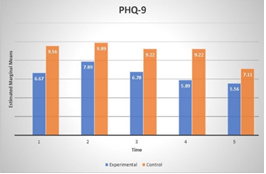
4. Patient Health Questionnaire - 9 (PHQ-9)
PHQ-9 showed a significant improvement over time (F(4, 32) =5,77, p=.001, η2 =.32), regardless of the group at 12-months. Interaction between group and time was not significant (p>.05).
5. World Health Organization Quality of Life (WHOQOL BREF)
WHOQOLDO analysis showed only significant main effect of TIME (F(4, 32) =3.27, p=.02, η2 =.29), for domain 1 (Physical Health). Further post-hoc comparisons did not show any significant differences over time.
Discussion
The present study aimed to investigate the efficacy of combining Blood Flow Restriction (BFR) with standard physical therapy (PT) exercises compared to PT alone in individuals with Ehlers-Danlos Syndrome (EDS). Our findings indicate significant improvements in several key outcomes, highlighting the potential of BFR as a therapeutic adjunct in this population.
Pain
The findings of this study reveal a notable reduction in pain scores for the PT-BFR group compared to those in the PT group. This observation prompts a deeper exploration into the potential mechanisms and clinical implications of BFR as a therapeutic strategy for managing pain in this population. One plausible explanation for the observed pain reduction in the BFR group involves the physiological effects induced by BFR during exercise [53]. BFR creates a controlled ischemic environment in the exercising limb. This environment stimulates metabolic stress and hormonal responses, which may contribute to pain modulation through several mechanisms: 1. Muscle Stabilization - EDS patients often experience musculoskeletal pain due to joint instability and weakened connective tissues. BFR has been shown to enhance muscle activation and improve muscle strength, potentially stabilizing joints and reducing pain associated with joint instability. 2. Neurophysiological Effects - BFR triggers neurophysiological responses that can modulate pain perception. It may influence pain pathways through mechanisms such as the release of endogenous opioids, alteration of nociceptive signaling, or modulation of central pain processing mechanisms. These effects could contribute to the observed decrease in pain scores among BFR participants. 3. Anti-inflammatory Effects - The localized hypoxic environment induced by BFR may also lead to anti-inflammatory responses. Inflammation is often a contributing factor to pain in EDS patients, and the reduction of inflammatory mediators through BFR-induced hypoxia could potentially alleviate pain symptoms. The findings of reduced pain scores in the PT-BFR group suggest potential clinical applications for BFR as a non-pharmacological intervention in pain management for EDS patients. Given the chronic nature of pain in EDS and the limitations of conventional treatments, BFR offers a promising avenue for improving pain outcomes with minimal adverse effects.
Muscle Strength and Volume
Our results revealed notable enhancements in knee extensor and flexor strength specifically in the experimental group that received PT-BFR compared to those who underwent standard PT exercises alone. This finding suggests that BFR may facilitate superior gains in muscle strength despite utilizing lower exercise intensities, which is crucial given the joint fragility commonly observed in individuals with EDS. Interestingly, while improvements in strength were observed predominantly in the left lower limb of the PT-BFR group, the right lower limb did not show statistically significant gains. This could be a potential representation of the right lower limb being considered the dominant limb and the musculature being recruited more often during daily functions. Unfortunately, we did not test for limb dominance in this study, so this is only speculation. Also due to the small number of participants it is possible that the study was underpowered, and the addition of more participants would have shown significant results as there did appear to be a trend towards significant improvement. This asymmetry warrants further investigation into potential underlying mechanisms or variations in response to BFR between limbs.
Ultrasonographic assessments of muscle volume demonstrated significant increases in the vastus lateralis and lateral head of the gastrocnemius among participants receiving PT-BFR. These findings align with previous literature indicating BFR's ability to induce muscle hypertrophy even at reduced training loads, offering a promising strategy to mitigate muscle atrophy commonly seen in EDS. The potential for muscle hypertrophy and associated strength gains offers a theoretical basis for improving joint stability, mobility, and function [54,55].
Quality of Life
Regarding QOL measures assessed by the WHOQOL-BREF, significant improvements were limited to Domain one (physical health) in the PT-BFR group. This domain encompasses aspects such as mobility, daily activities, and pain, reflecting the tangible benefits observed in muscle strength and pain reduction. In contrast, domains assessing psychological health, social relationships, and environment did not show significant changes between groups, indicating that while BFR may enhance physical health-related quality of life domains, broader psychosocial impacts may require additional therapeutic considerations.
Clinical Implications and Future Directions
The findings of this study underscore the potential of BFR as an innovative adjunct to conventional PT in managing musculoskeletal deficits associated with EDS. By promoting muscle hypertrophy and strength gains at lower exercise intensities, BFR offers a safe and effective alternative to traditional resistance training methods that may exacerbate joint instability and tissue fragility in this vulnerable population.
Future research should explore optimal BFR protocols tailored to individual EDS subtypes and severity levels to maximize therapeutic benefits. Long-term follow-up studies are warranted to assess the sustainability of BFR-induced improvements in muscle strength, pain management, and overall quality of life. Additionally, investigating the underlying physiological mechanisms driving asymmetrical strength gains between limbs could inform personalized rehabilitation strategies for individuals with EDS.
Conclusion
In conclusion, this study represents a first step in the potential for stimulating research to advance the management of EDS. While only a pilot this study offers insight into a promising alternative to traditional rehabilitation approaches. The improvements observed in muscle strength, volume, outcomes, and overall well-being in the PT-BFR group underscore the transformative potential of this innovative intervention. By addressing the multifaceted challenges of EDS through personalized and effective rehabilitation strategies like PT-BFR, this research paves the way for improving long-term outcomes and enhancing quality of life for individuals living with this complex syndrome.
Limitations
Despite efforts to rigorously design and execute this randomized controlled trial (RCT) investigating the effects of BFR in individuals with EDS, several limitations must be acknowledged. One of the primary limitations of this study is the small sample size of 18 participants. While efforts were made to recruit a diverse group of individuals with EDS, the generalizability of the findings may be limited due to the small number of participants. A larger sample size would have provided more statistical power and potentially more robust conclusions. EDS is a heterogeneous disorder with various subtypes and manifestations. Despite efforts to include participants with different EDS subtypes, the study population does not fully represent the entire spectrum of EDS. Variations in symptoms and severity among participants could affect the generalizability of the findings to all individuals with EDS. Despite randomization, there may be unidentified biases or confounding variables that could have influenced the study results. Factors such as concurrent treatments, lifestyle habits, or environmental influences were not fully controlled for and could have impacted the outcomes observed. As can be seen it appears that the BFR group and PT group showed differences on several outcome measures at baseline which may have affected the results. Future research should therefore prioritize larger, multicenter trials with extended follow-up periods to elucidate the durability of PT-BFR effects over time and across diverse EDS phenotypes.
Contributors
JZL was involved in all aspects of the study from design and data collection through manuscript submission. TM, KS, SV, participated in data collection and preparation of the manuscript, MPF performed the statistical analysis and assisted in manuscript preparation. All authors had full access to the data in the study and had final responsibility for the decision to submit for publication. We declare no competing interests.
Acknowledgements
This study was funded by an internal grant from the University of Rhode Island. This work could not have been completed without the tireless efforts of Nicole Asquino, Daniel DeLuca, Kristen English, Tzipporah Kapilevich, and Jimmy Sorel.
Conflict of Interests:
The authors declare that there are no conflicts of interest.
References
Gensemer, C., Burks, R., Kautz, S., Judge, D. P., Lavallee, M., Norris, R. A., (2021). Hypermobile Ehlers-Danlos syndromes: Complex phenotypes, challenging diagnoses, and poorly understood causes. Developmental dynamics: an official publication of the American Association of Anatomists. 250(3), 318–344.View
Ritelli, M., Colombi, M., (2020). Molecular Genetics and Pathogenesis of Ehlers-Danlos Syndrome and Related Connective Tissue Disorders. Genes. 11(5), 547.View
Malfait, F., Wenstrup, R. J., De Paepe, A., (2010). Clinical and genetic aspects of Ehlers-Danlos syndrome, classic type. Genet Med. Oct; 12(10):597-605.View
National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Committee on Selected Heritable Disorders of Connective Tissue and Disability. Selected Heritable Disorders of Connective Tissue and Disability. Wedge RA, Cartaxo T, Spicer CM, Volberding PA, editors. Washington (DC): National Academies Press (US); 2022 Jul 8. PMID: 36223440.View
Malfait, F., Francomano, C., Byers, P., Belmont, J., Berglund, B., Black, J., Bloom, L., Bowen, J. M., Brady, A. F., Burrows, N. P., Castori, M., Cohen, H., Colombi, M., Demirdas, S., De Backer, J., De Paepe, A., Fournel-Gigleux, S., Frank, M., Ghali, N., Giunta, C., Grahame, R., Hakim, A., Jeunemaitre, X., Johnson, D., Juul-Kristensen, B., Kapferer-Seebacher, I., Kazkaz, H., Kosho, T., Lavallee, M. E., Levy, H., Mendoza-Londono, R., Pepin, M., Pope, F. M., Reinstein, E., Robert, L., Rohrbach, M., Sanders, L., Sobey, G. J., Van Damme, T., Vandersteen, A., van Mourik, C., Voermans, N., Wheeldon, N., Zschocke, J., Tinkle, B., (2017). The 2017 international classification of the Ehlers Danlos syndromes. Am J Med Genet C Semin Med Genet. Mar 175(1):8-26. doi: 10.1002/ajmg.c.31552. PMID: 28306229.View
Demmler, J. C., Atkinson, M. D., Reinhold, E. J., Choy, E., Lyons, R. A., Brophy, S. T., (2019). Diagnosed prevalence of Ehlers-Danlos syndrome and hypermobility spectrum disorder in Wales, UK: a national electronic cohort study and case control comparison. BMJ Open. Nov 4;9(11):e031365. doi: 10.1136/bmjopen-2019-031365. PMID: 31685485; PMCID: PMC6858200.View
Laferrier, J. Z., Muldowney, K., Muldowney, K., (2018). A Novel Exercise Protocol for Individuals with Ehlers Danlos Syndrome: A Case Report. J Nov Physiother. 8: 382. View
Yew, K. S., Kamps-Schmitt, K. A., Borge, R., (2021). Hypermobile Ehlers-Danlos Syndrome and Hypermobility Spectrum Disorders. American family physician. 103(8), 481 492.View
Badauy, C. M., Gomes, S. S., Sant'Ana Filho, M., Chies, J. A., (2007). Ehlers-Danlos syndrome (EDS) type IV: review of the literature. Clinical oral investigations. 11(3), 183–187.View
van Dijk, F. S., Ghali, N., Chandratheva, A., (2024). Ehlers-Danlos syndromes: importance of defining the type. Practical neurology. 24(2), 90–97.View
Gilliam, E., Hoffman, J. D., Yeh, G., (2020). Urogenital and pelvic complications in the Ehlers-Danlos syndromes and associated hypermobility spectrum disorders: A scoping review. Clin Genet. 97(1):168-78. Epub 20190901. doi: 10.1111/ cge.13624. PubMed PMID: 31420870; PMCID: PMC6917879. View
Henderson, F. C., Sr., Austin, C., Benzel, E., Bolognese, P., Ellenbogen, R., Francomano, C. A., Ireton, C., Klinge, P., Koby, M., Long, D., Patel, S., Singman, E. L., Voermans, N. C., (2017). Neurological and spinal manifestations of the Ehlers Danlos syndromes. Am J Med Genet C Semin Med Genet. 175(1):195-211. Epub 20170221. doi: 10.1002/ajmg.c.31549. PubMed PMID: 28220607.View
Song, B., Yeh, P., Nguyen, D., Ikpeama, U., Epstein, M., Harrell, J., (2020). Ehlers-Danlos Syndrome: An Analysis of the Current Treatment Options. Pain physician. 23(4), 429–438.View
Leganger, J., Søborg, M. L., Farholt, S., Lund, A. M., Rosenberg, J., Burcharth, J., (2016). [Ehlers-Danlos syndrome]. Ugeskr Laeger. Apr 25; 178(17):V01160014. Danish. PMID: 27136954.View
Riley, B., (2020). The Many Facets of Hypermobile Ehlers Danlos Syndrome. J Am Osteopath Assoc. Jan 1; 120(1):30-32. doi: 10.7556/jaoa.2020.012. PMID: 31904772.View
Patel, M., Khullar, V., (2021). Urogynaecology and Ehlers Danlos syndrome. Am J Med Genet C Semin Med Genet. Dec; 187(4): 579-585. doi: 10.1002/ajmg.c.31959. Epub 2021 Nov 20. PMID: 34799982.View
Kumskova, M., Flora, G. D., Staber, J., Lentz, S. R., Chauhan, A. K., (2023). Characterization of bleeding symptoms in Ehlers Danlos syndrome. J Thromb Haemost. Jul; 21(7):1824-1830. doi: 10.1016/j.jtha.2023.04.004. Epub 2023 Apr 17. PMID: 37179130.View
Rachapudi, S. S., Laylani, N. A., Davila-Siliezar, P. A., Lee, A. G., (2023). Neuro-ophthalmic manifestations of Ehlers-Danlos syndrome. Curr Opin Ophthalmol. Nov 1; 34(6):476-480. doi: 10.1097/ICU.0000000000001002. Epub 2023 Aug 30. PMID: 37729660.View
Schubart, J. R., Mills, S. E., Schaefer, E. W., Bascom, R., Francomano, C. A., (2022). Longitudinal analysis of symptoms in the Ehlers-Danlos syndromes. Am J Med Genet A. Apr; 188(4):1204-1213. doi: 10.1002/ajmg.a.62640. Epub 2022 Jan 7. PMID: 34994522; PMCID: PMC10433231.View
Olubajo, F., Kaliaperumal, C., Choudhari, K. A., (2020). Vascular Ehlers-Danlos Syndrome: Literature review and surgical management of intracranial vascular complications. Clin Neurol Neurosurg. Jun; 193:105775. doi: 10.1016/j. clineuro.2020.105775. Epub 2020 Mar 3. PMID: 32197145.View
Montero-Fernández, N., Serra-Rexach, J. A., (2013). Role of exercise on sarcopenia in the elderly. European journal of physical and rehabilitation medicine. 49(1), 131–143.View
Fragala, M. S., Cadore, E. L., Dorgo, S., Izquierdo, M., Kraemer, W. J., Peterson, M. D., Ryan, E. D., (2019). E. D. Resistance Training for Older Adults: Position Statement from the National Strength and Conditioning Association. Journal of strength and conditioning research. 33(8), 2019–2052. https:// doi.org/10.1519/JSC.0000000000003230.View
Angulo, J., El Assar, M., Álvarez-Bustos, A., Rodríguez-Mañas, L., (2020). Physical activity and exercise: Strategies to manage frailty. Redox biology. 35, 101513. https://doi.org/10.1016/j. redox.2020.101513.View
Yoon, D. H., Lee, J. Y., Song, W., (2018). Effects of Resistance Exercise Training on Cognitive Function and Physical Performance in Cognitive Frailty: A Randomized Controlled Trial. The journal of nutrition, health & aging. 22(8), 944–951. https://doi.org/10.1007/s12603-018-1090-9. View
American College of Sports Medicine. (2009). American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Medicine and science in sports and exercise. 41(3), 687–708. https://doi.org/10.1249/ MSS.0b013e3181915670.View
Jørgensen, S. L., Kierkegaard-Brøchner, S., Bohn, M. B., et al. (2023). Effects of blood-flow restricted exercise versus conventional resistance training in musculoskeletal disorders—a systematic review and meta-analysis. BMC Sports Sci Med Rehabil . 15, 141. https://doi.org/10.1186/s13102-023-00750-z. View
Libardi, C. A., Chacon-Mikahil, M. P., Cavaglieri, C. R., Tricoli, V., Roschel, H., Vechin, F. C.,Conceicao, M. S., Ugrinowitsch, C., (2015). Effect of concurrent training with blood flow restriction in the elderly. Int J Sports Med. 36:395–9.View
Vechin, F. C., Libardi, C. A., Conceicao, M. S., Damas, F. R., Lixandrao, M. E., Berton, R. P., Tricoli, V. A., Roschel, H. A., Cavaglieri, C. R., Chacon-Mikahil, M. P., Ugrinowitsch, C., (2015). Comparisons between low-intensity resistance training with blood flow restriction and high-intensity resistance training on quadriceps muscle mass and strength in elderly. J Strength Cond Res. 29:1071–6.View
Patterson, S. D., Hughes, L., Warmington, S., Burr, J., Scott, B. R., Owens, J., Abe, T., Nielsen, J. L., Libardi, C. A., Laurentino, G., Neto, G. R., Brandner, C., Martin-Hernandez, J., & Loenneke, J., (2019). Blood Flow Restriction Exercise: Considerations of Methodology, Application, and Safety. Front Physiol. May 15; 10:533. doi: 10.3389/fphys.2019.00533. Erratum in: Front Physiol. 2019 Oct 22; 10:1332. doi: 10.3389/ fphys.2019.01332. PMID: 31156448; PMCID: PMC6530612.View
Fan, Y., Bai, D., Cheng, C., Tian, G., (2023). The effectiveness and safety of blood flow restriction training for the post-operation treatment of distal radius fracture. Ann Med. 55(2):2240329. doi: 10.1080/07853890. 2240329. PMID: 37505919; PMCID: PMC10392265.View
Jønsson, A. B., Krogh, S., Laursen, H. S., Aagaard, P., Kasch, H., Nielsen, J. F., (2024). Safety and efficacy of blood flow restriction exercise in individuals with neurological disorders: A systematic review. Scand J Med Sci Sports. Jan; 34(1):e14561. doi: 10.1111/sms.14561. PMID: 38268066. View
Vinolo-Gil, M. J., García-Campanario, I., Estebanez-Pérez, M. J., Pastora-Bernal, J. M., Rodríguez-Huguet, M., & Martín Vega, F. J., (2023). Blood Flow Restriction in Oncological Patients: Advantages and Safety Considerations. Healthcare (Basel). Jul 19; 11(14):2062. doi: 10.3390/healthcare11142062. PMID: 37510502; PMCID: PMC10379018.View
Minniti, M. C., Statkevich, A. P., Kelly, R. L., Rigsby, V. P., Exline, M. M., Rhon, D. I., & Clewley, D., (2020). The Safety of Blood Flow Restriction Training as a Therapeutic Intervention for Patients with Musculoskeletal Disorders: A Systematic Review. Am J Sports Med. Jun; 48(7):1773-1785. doi: 10.1177/0363546519882652. Epub 2019 Nov 11. PMID: 31710505.View
Anderson, K. D., Rask, D. M. G., Bates, T. J., & Nuelle, J. A. V., (2022). Overall Safety and Risks Associated with Blood Flow Restriction Therapy: A Literature Review. Mil Med. Aug 25; 187(9-10):1059-1064. doi: 10.1093/milmed/usac055. PMID: 35284924.View
Lixandrao, M. E., Ugrinowitsch, C., Laurentino, G., Libardi, C. A., Aihara, A. Y., Cardoso, F. N., Tricoli, V., & Roschel, H., (2015). Effects of exercise intensity and occlusion pressureafter 12 weeks of resistance training with blood-flow restriction. Eur J Appl Physiol. 115:2471–80.View
Lixandrão, M. E., Ugrinowitsch, C., Berton, R., Vechin, F. C., Conceição, M. S., & Damas, F., et al. (2018). Magnitude of Muscle Strength and Mass Adaptations Between High-Load Resistance Training Versus Low-Load Resistance Training Associated with Blood-Flow Restriction: A Systematic Review and Meta-Analysis. Sports medicine (Auckland, NZ). 48(2):361–78.View
Segal, N., Davis, M. D., & Mikesky, A. E., (2015). Efficacy of blood flow-restricted low-load resistance training for quadriceps strengthening in men at risk of symptomatic knee osteoarthritis. Geriatr Orthop Surg Rehabil. 6:160–7.View
Segal, N. A., Williams, G. N., Davis, M. C., Wallace, R. B., & Mikesky, A. E., (2015). Efficacy of blood flow-restricted, low-load resistance training in women with risk factors for symptomatic knee osteoarthritis. PM R. 7:376–84.View
Bryk, F. F., Dos Reis, A. C., Fingerhut, D., Araujo, T., Schutzer, M., Cury Rde, P., Duarte, A. Jr, & Fukuda, T. Y., (2016). Exercises with partial vascular occlusion in patients with knee osteoarthritis: a randomized clinical trial. Knee Surg Sports Traumatol Arthrosc. 24:1580–6.View
Giles, L., Webster, K. E., McClelland, J., & Cook, J. L., (2017). Quadriceps strengthening with and without blood flow restriction in the treatment of patellofemoral pain: a double blind randomized trial. Br J Sports Med. 0:1–8.View
Tennent, D. J., Hylden, C. M., Johnson, A. E., Burns, T. C., Wilken, J. M., & Owens, J. G., (2017). Blood flow restriction training after knee arthroscopy: a randomized controlled pilot study. Clin J Sport Med. 27:245–52.View
Castle, J. P., Tramer, J. S., Turner, E. H. G., Cotter, D., McGee, A., Abbas, M. J., Gasparro, M. A., Lynch, T. S., & Moutzouros, V., (2023). Survey of blood flow restriction therapy for rehabilitation in Sports Medicine patients. J Orthop. Mar 13; 38:47-52. Doi: 10.1016/j.jor.2023.03.007. PMID: 36969302; PMCID: PMC10030811.View
Perera, E., Zhu, X. M., Horner, N. S., Bedi, A., Ayeni, O. R., & Khan, M., (2022). Effects of Blood Flow Restriction Therapy for Muscular Strength, Hypertrophy, and Endurance in Healthy and Special Populations: A Systematic Review and Meta-Analysis. Clin J Sport Med. Sep 1; 32(5):531-545. doi: 10.1097/JSM.0000000000000991. Epub 2021 Nov 29. PMID: 36083329.View
Mañago, M. M., Kimbrell, K., Hager, E. R., Dwight, H., Owens, J., & Bade, M., (2022). Clinical use of blood flow restriction in people with neurologic conditions: a cross-sectional survey. J Phys Ther Sci. Apr; 34(4):275-283. doi: 10.1589/jpts.34.275. Epub 2022 Apr 8. PMID: 35400831; PMCID: PMC8989480.View
Hedt, C., McCulloch, P. C., Harris, J. D., & Lambert, B. S., (2022). Blood Flow Restriction Enhances Rehabilitation and Return to Sport: The Paradox of Proximal Performance. Arthrosc Sports Med Rehabil. Jan 28; 4(1):e51-e63. doi: 10.1016/j. asmr.2021.09.024. PMID: 35141536; PMCID: PMC8811501.View
Rolnick, N., Kimbrell, K., de Queiros, V., (2023). Beneath the cuff: Often overlooked and under-reported blood flow restriction device features and their potential impact on practice-A review of the current state of the research. Front Physiol. Mar 30; 14:1089065. doi: 10.3389/fphys.2023.1089065. PMID: 37064884; PMCID: PMC10099250.View
PTSii Tourniquet System. (2024). Accessed June 5, https:// www.delfimedical.com/pts-tourniquet-system/.View
Keller, S., Bann, C. M., Dodd, S. L., Schein, J., Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004 Sep-Oct; 20(5):309-18. doi: 10.1097/00002508 200409000-00005. PMID: 15322437.View
Hirano, M., Katoh, M., Gomi, M., & Arai, S., (2020). Validity and reliability of isometric knee extension muscle strength measurements using a belt-stabilized hand-held dynamometer: a comparison with the measurement using an isokinetic dynamometer in a sitting posture. J Phys Ther Sci. Feb; 32(2):120-124. doi: 10.1589/jpts.32.120. Epub 2020 Feb 14. PMID: 32158074; PMCID: PMC7032982.View
Gilbody, S., Richards, D., Brealey, S., & Hewitt, C., (2007). Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. J Gen Intern Med. 22(11):1596-602. doi: 10.1007/s11606-007-0333-y. Epub 2007 Sep 14. PMID: 17874169; PMCID: PMC2219806.View
Naruse, M., Trappe, S., & Trappe, T. A., (2022). Human skeletal muscle size with ultrasound imaging: a comprehensive review. J Appl Physiol (1985). 1;132(5):1267-1279. doi: 10.1152/ japplphysiol.00041.2022. Epub 2022 Mar 31. PMID: 35358402; PMCID: PMC9126220.View
Skevington, S., Lotfy, M., & O'Connell, K., (2004). The World Health Organization's WHOQOL-BREF quality of life assessment: Psychometric properties and results of the international field trial. A report from the WHOQOL Group. Qual Life Res. 13: 299–310.View
Karanasios, S., Lignos, I., Kouvaras, K., Moutzouri, M., & Gioftsos, G., (2023). Low-Intensity Blood Flow Restriction Exercises Modulate Pain Sensitivity in Healthy Adults: A Systematic Review. Healthcare (Basel). 2; 11(5):726. doi: 10.3390/healthcare11050726. PMID: 36900731; PMCID: PMC10000465.View
Toprak Celenay, S., & Ozer Kaya, D., (2017). Effects of spinal stabilization exercises in women with benign joint hypermobility syndrome: a randomized controlled trial. Rheumatol Int. 37(9):1461-1468. doi: 10.1007/s00296-017-3713-6. Epub 2017 Mar 30. PMID: 28361275.View
Monti, E., Tagliaferri, S., Zampieri, S., Sarto, F., Sirago, G., Franchi, M. V., Ticinesi, A., Longobucco, Y., Adorni, E., Lauretani, F., Von Haehling, S., Marzetti, E., Calvani, R., Bernabei, R., Cesari, M., Maggio, M., & Narici, M. V., (2023). Effects of a 2-year exercise training on neuromuscular system health in older individuals with low muscle function. J Cachexia Sarcopenia Muscle. 14(2):794-804. doi: 10.1002/jcsm.13173. Epub 2023 Jan 28. PMID: 36708273; PMCID: PMC10067485.View