Journal of Rehabilitation Practices and Research, Volume 5 (2024), Article ID: JRPR-151
https://doi.org/10.33790/jrpr1100151Research Article
The Effects of Patient Instructions on Optokinetic Testing During a Vestibular Test Battery
Cara Makuta Tolan1*, Devon Suiter2
1*Assistant Professor, Department of Communication Sciences & Disorders, Commonwealth University of Pennsylvania - Bloomsburg, Pennsylvania Tolan, United States.
2Clinical Audiologist, Alexander T. Augusta Military Center, Fort Belvoir, Virginia, United States.
Corresponding Author Details: Cara Makuta Tolan, Au.D., Ph.D., Assistant Professor, Department of Communication Sciences & Disorders, Commonwealth University of Pennsylvania Bloomsburg, 36 Centennial Hall, 400 East Second St. Bloomsburg, PA 17815-1301 United States.
Received date: 26th September, 2024
Accepted date: 28th October, 2024
Published date: 30th October, 2024
Citation: Tolan, C. M., & Suiter, D., (2024). The Effects of Patient Instructions on Optokinetic Testing During a Vestibular Test Battery. J Rehab Pract Res, 5(1):151.
Copyright: ©2024, This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Diagnostic vestibular testing relies on established clinical standards and best clinical practice guidelines. Currently, there are no specific best practices regarding instructing vestibular patients. Understanding that a stare OKN is a subcortical reflexive response, it is assumed that OKN patient instructions should not influence the stare OKN gain scores. Previous research is limited on the impact of patient OKN instructions compared to no instructions on stare OKN gain scores. This study aimed to examine the effects of patient instructions (with and without) on Optokinetic Nystagmus (OKN) gain measures.
OKN gain scores were measured and recorded from 35 participants. Data collection included recording OKN gain scores with and without OKN instructions. OKN instructions were verbally stated to the subjects, and gain scores were measured as the OKN targets moved at 40°/sec, 60°/sec, and 80°/sec in the rightward and leftward directions. A repeated measure analysis of variance (ANOVA) measured the overall differences between the two test conditions, OKN measurement with and without patient instructions. Study findings did not yield any statistically significant effect on OKN instruction. However, a significant relationship was identified between the velocity/speed of the OKN targets and OKN gain scores. Specifically, the higher the velocity of the OKN target, the lower the observed OKN gain score.
It was found that OKN gains were not influenced by the completion of OKN instructions. However, an inverse relationship was observed between OKN target velocity and OKN gain scores. Overall gain scores decreased with the increase in OKN target velocity/speed. These findings underscore the importance of considering the velocity of the OKN target when interpreting OKN gain scores, and they suggest that the completion of OKN instructions does not significantly affect OKN gains.
Keywords: Videonystagmography (VNG), OKN gain scores, OKN target velocity/speed, Diagnostic Balance Testing, Vestibular Disorders
Introduction
The maintenance of balance and equilibrium depends on integrating three critical sensory systems: vision, proprioception, and vestibular function. If a weakness or dysfunction occurs in any of these systems, compromised balance and other symptoms, such as vertigo and dizziness, can occur [1]. Therefore, understanding the normal function and identifying the dysfunction in these systems is essential for patient care.
An essential component of normal vestibular function in an individual is gaze stabilization. The two common types of gaze stabilization are the vestibular-ocular reflex (VOR) and optokinetic nystagmus (OKN). VOR is an eye movement produced by rotation and translations of the head and body in space to stabilize vision [2]. Conversely, OKN is a rhythmic oscillation of the eye produced when a visual field moves past a stationary subject [2]. Vestibular testing has evolved over the years, allowing for accurate assessment of gaze stabilization in patients who exhibit or report dizziness and disequilibrium.
Diagnostic vestibular testing relies on established clinical standards and best clinical practice guidelines. However, specific best clinical practice guidelines regarding instructing vestibular patients do not currently exist. Today's clinical standards include a concrete overall test battery to ensure a proper diagnosis but no specific test instructions for balance patients. Clinicians commonly utilize the recording of eye movements to examine vestibular function. The most direct and practical way to analyze and evaluate the peripheral vestibular system is through ocular motility testing [1]. Gaze stabilization assessment is performed by recording OKN responses with videonystagmography (VNG), which allows the clinician to examine eye movement in three planes - horizontal, vertical, and torsional Katz et al., [3]. OKN responses require an entire field of visual stimulation to be elicited. Moreover, OKN stimulation must cover at least 90% of the patient's visual field. This orientation of OKN stimulation should make it nearly impossible for a person to suppress their OKN reflex [4].
A study conducted in 1968 by Honrubia suggested that clinicians can elicit two types of OKN responses through only patient instruction. Look nystagmus, also known as pursuit OKN, is elicited by specific instructions to focus on a particular target, requiring the subject to actively attend the test [5]. This type of response is always cortical because the subjects actively think about the task throughout the session [6]. Stare nystagmus is a reflexive response when a subject passively follows a moving visual field [5]. Recorded responses are considered subcortical or reflexive [6]. Understanding that there are two different types of responses and the reflexive nature of stare nystagmus, it questioned how patient instructions influence OKN test results. Jacobson and Shepard [1] suggested that the consistency, reliability, and validity of ONK scores can be affected by patient instructions. Therefore, this study aimed to investigate the effects of OKN instructions on stare OKN gains. This study did not examine look nystagmus because it is established that the response is cortical, meaning that the patients are using their attention to elicit better results. The study examined the effects of detailed, scripted OKN instructions versus no instructions on OKN gain in subjects with normally functioning vestibular systems. This study was designed to investigate the impact of patient instructions versus omitting instructions during OKN tests and to explore the instruction's influence on clinical utility.
With the understanding that a stare OKN is a subcortical reflexive response, the assumption is made that there would be no difference in OKN scores between patients who receive scripted stare nystagmus instructions compared to participants who do not receive instructions. The OKN patient instructions should not influence the stare OKN gain scores. Previous research is limited on the impact of patient OKN instructions compared to no instructions on stare OKN gain scores. The goal was to understand the influence of patient instructions on stare OKN gain measurements with the potential of outlining some clinical practice guidelines for OKN patient instructions.
Materials & Methods
The primary goal of this quantitative research was to investigate the impact of instructions on ONK gain results. The study design was structured to explore the relationship between variables in a predictable pattern for a group of individuals. The independent variables of this study included OKN instruction versus no instruction and the velocity of the OKN targets (40°/sec, 60°/sec, and 80°/sec). The dependent variables measured were overall OKN gain scores. The study aimed to determine whether patient instructions can help clinicians obtain better stare nystagmus outcomes.
The study recruited 35 participants (four males and 31 females) ranging in age from 18 to 30 (mean age 21.8). The study protocol implemented a rigorous screening process to ensure the reliability and validity of our results. Exclusion criteria included a history of ear disease, vestibular complaints, vertiginous complaints, otologic surgery, hearing impairment (sensorineural and conductive hearing loss), diagnosis of migraine, issues in visual acuity (other than contact lenses or glasses), brain injury, neurologic deficits, or abnormal results in VNG or audiologic battery. Before testing, screening for gaze-evoked nystagmus, spontaneous nystagmus, random saccades, and smooth pursuit was completed. All study participants had to be within normal limits relative to age. Subjects were instructed to stop consuming caffeine, alcohol, and nicotine and to avoid taking vestibular suppressant medications 24 hours before testing. Subjects were also asked not to wear face or eye makeup to reduce the risk of recording artifacts in video-ocular tracings. All study participants scored less than 15 on the Dizziness Handicap Inventory (DHI). The DHI is a subjective measure of a person's perceived handicap; scores under 15 suggest little to no self-perceived handicap. The above-described vestibular screening protocol and DHI eliminated participants with any unknown vestibular dysfunction, which could affect OKN gain scores based on an underlying pathology.
Testing was performed using a state-of-the-art NeuroKinetics I-Portal (NOTC) Rotary Chair, an earth vertical rotational device capable of measuring the VOR from 0.01 to 1.28 Hz at velocities up to 300°/s (Neuro Kinetics, Inc., Pittsburgh, PA). This rotary chair uses the Vest 7.5 software housed in a light-tight circular room. Patients' eye movements were recorded using VNG goggles connected to the I-Portal 3.2 software. We ensured the high standards and precision of our study by conducting equipment calibration annually and performing biological checks of the rotary chair and VEST software daily to confirm that instrumentation problems did not occur, preserving the internal validity of this study.
Study participants were seated in the rotational chair and secured with the proper coupling of the rotation stimulus to the subject's head, ensuring that the semicircular canals were in the correct plane of rotation. The chair was calibrated before each trial, as recommended by the manufacturer. Eye responses were measured using 4D video-oculography with a binocular eye-tracking device attached via goggles over the eyes. The goggles were calibrated on every participant before any data collection. Following calibration, a brief NOTC ocular motor test battery was completed. The tests included horizontal smooth pursuit testing and saccadic testing, which offered one more layer of screening to identify any nystagmus or abnormal eye jerks. Any identification of abnormalities on the ocular motor testing could suggest a peripheral vestibular weakness or central etiology, which would exclude the participant from the study. Therefore, to proceed with the study's testing protocol, participants were required to perform within the normal range for smooth pursuit and the saccade tests.
Selected study subjects were given six conditions of OKN target velocity. The OKN targets were presented at the following velocities or speeds: 40°/sec, 60°/sec, and 80°/sec, moving in the rightward and leftward directions. All participants had the same testing protocol and requirements. The OKN targets were rotated at a constant speed (40°/ sec, 60°/sec, or 80°/sec) for 30 seconds in the rightward direction and 30 seconds in the leftward direction. Eye recordings were made once the targets began rotating, and recording ended when the targets were no longer moving. After the three testing conditions, participants were given the scripted instructions for OKN testing. Study participants heard the script before each of the three testing conditions; therefore, the participants listened to the OKN instructions three times.
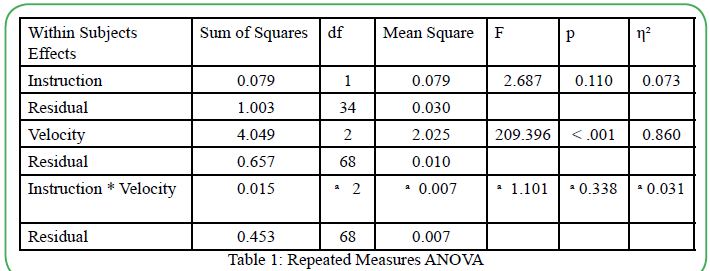
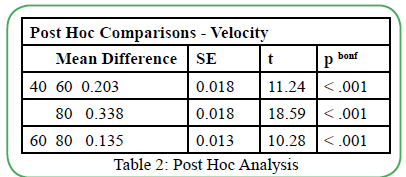
The collected OKN gain data was analyzed in "Jeffreys's Amazing Statistics Program," known as JASP. Due to the OKN gain interval data, a repeated measures ANOVA was used to analyze the withinsubject group design. A significant difference in OKN gain scores was observed between the testing velocities, and a post-hoc analysis was completed using a Bonferroni correction. The post-hoc analysis revealed a significant difference between the velocity of the moving OKN targets and OKN gain scores. The repeated measures ANOVA examined the differences between the two test conditions (with instructions and without instructions) while accounting for withingroup variability and overall error variability. The repeated measures ANOVA calculated any significant differences between instruction vs. no instruction, OKN gain, and velocity of the moving OKN targets.
Results
The results of the repeated measured ANOVA denied any significant effect of OKN instructions on the OKN gains, F (1,34) =2.687; p=0.110. The main impact of OKN velocity was statistically significant F (2, 68) = 209.396; p<0.01. The main effect (η² = 0.860) revealed a practical significance to this study, which means that similar results are suspected if the sample size were to increase. Interestingly, the analysis showed no significant interaction between the type of instruction (with or without) and OKN gain scores (F (2, 68) = 1.101; p=0.338). Mauchly's test of sphericity indicated that sphericity was violated (p<0.05). However, Mauchly's sphericity test is known to overestimate and over-assume the effect of small sample sizes when dealing with small sample sizes. These results are displayed in Table 1. Since the results yielded a statistical significance in the main effect of OKN target velocity, a post-hoc analysis with a Bonferroni correction revealed statistical significance between all OKN velocity testing conditions. The results can be found in Table 2.
Discussion
This study examined the effects of patient instructions on stare OKN gain scores in subjects with normal vestibular function. Test results denied any significant difference between giving a patient OKN instructions and not giving them instructions. It was observed that the OKN gain scores are not directly affected by providing patients with instructions on performing OKN tests. Therefore, based on the findings from this study, patient instructions will not influence the OKN gain measurements. However, a significant difference was observed between OKN gain scores and the OKN testing speeds or target velocities. The study found that OKN gain scores decreased as the speed of the OKN target velocity increased. This finding was supported by Jacobson & Shephard [1].
The results of this study denied any significant difference between patient instructions and OKN gain score. The clinical implication of this finding suggests that clinicians can expect similar stare OKN gain responses whether patient instructions are given or omitted, especially for those patients with a normally functioning vestibular system. Knowing that OKN gain scores are not directly affected by OKN patient instructions, clinicians can feel confident in the OKN test results regardless of testing instructions.
The findings of this study implore the following logical questions. First, are OKN patient instructions necessary to complete OKN testing? Second, what does the omission of OKN instructions offer to clinical practice? According to the study's findings, 'no' OKN patient instructions are unnecessary for OKN test completion. It is important to note that this study was conducted on participants with normal vestibular function. This study cannot confirm that the findings will translate to patients with compromised vestibular function. Therefore, good clinical decision-making is advised. Complete generalization from normal vestibular function to vestibular dysfunction regarding OKN gain responses and patient instruction influences is strongly cautioned against. The determination to completely omit OKN patient instructions should be left up to the clinician's preference and judgment, which will be guided by the patient's ability and needs during testing. Removing OKN patient instructions offers the potential for some clinical time savings. The time saved by omitting OKN instruction per patient is a small amount of time. However, these few seconds can add up throughout the days, weeks, months, and even years. This additional clinic time, which would result from time saved, can be allotted to other areas of clinical operations, patient management, and other service delivery.
The other study finding, which suggests noteworthy clinical implication, was the significant difference observed between OKN gain scores and the OKN testing speeds or target velocities. The study found an inverse relationship between OKN gain scores and OKN target velocities. It was observed that OKN gain scores will decrease as the OKN target velocity increases. From this finding, it is argued that more than two or three- OKN testing speeds/velocities are needed to offer a complete diagnostic assessment of OKN performance. OKN results correlate to VOR function or can support the identification of a complicated vestibular dysfunction. In that case, a clinical examination should include additional OKN target speeds/velocities, which can offer a more comprehensive evaluation of the VOR and vestibular function, especially at higher rates where the OKN gains were observed to reduce. The clinical time saved by removing OKN patient instruction allows time for testing more (higher velocity) OKN testing targets.
A limitation of this study is that it needs to provide information on other factors that might contribute to a patient's OKN gain scores. This study examined the OKN gain scores of young, healthy adults without vestibular disorders. A future study is recommended to increase the subject's age and explore a population with vestibular dysfunction. Another study limitation was the population size. This study utilized a sample of 35 subjects. A larger sample size is also recommended as it could yield or alter the statistical findings. Additionally, the presentation order of OKN target velocities was the same for all 35 participants. A limitation of this study is the potential carry-over and test-practice effects, which could influence the participants' OKN gain scores. Randomization and subject counterbalancing are suggested for future research.
The researchers acknowledge that it is difficult to generalize this study's results to patients with impaired or disordered vestibular systems. Although OKN instructions were not found to influence individuals with normally functioning systems, instructions might be required or even assist in obtaining improved OKN gain scores in patients with impaired vestibular systems and other compromised conditions, impacting OKN test performance.
In conclusion, this study found that the type of OKN instruction, whether given or omitted, did not significantly affect stare OKN gain scores. This suggests that omitting OKN patient instructions can save valuable clinical time, especially in patients with suspected normal vestibular function. The potential to save some clinical time, albeit a small amount, is a significant clinical implication of this study.
Upon closer examination of OKN gain scores and velocity measurements, an inverse relationship was observed between OKN gain scores and OKN target velocities. This finding agreed with the findings of Jacobson & Shepard (2016), which state that gain scores decrease as target velocities increase. Therefore, with time saved via the omission of OKN instruction, the clinical utility would be improved by adding OKN target velocity or velocities. This study suggests that testing more (higher/faster) OKN target velocities/ speeds to evaluate VOR function further and identify high-frequency vestibular disorders should be considered.
Conflicts of Interest:
The authors declare that they have no competing interests.
List of Abbreviations
A Repeated Measure Analysis of Variance (ANOVA)
Dizziness Handicap Inventory (DHI)
NeuroKinetics I-Portal Rotary Chair (NOTC)
Vestibular-Ocular Reflex (VOR)
Optokinetic Nystagmus (OKN)
Vestibular-Ocular Reflex (VOR)
Videonystagmography (VNG)
Acknowledgments
The researchers would like to thank the Commonwealth University of Pennsylvania – Bloomsburg, as this was the site of this study’s completion. The researchers would also like to acknowledge Dr Michael McKenna, who assisted with data collection and supported the researchers throughout the project.
References
Jacobson, G.P. & Shepard, N.T. (2016). Balance function assessment and management. San Diego, CA.View
Kenari, K., Sakamoto, K. & Kaneko, H. (2017). Effect of visual attention on the properties of optokinetic nystagmus. PLoS ONE. 12(4), e0175453. View
Katz, J., Chasin, M., English, K., Hood, L.J. & Tillery, K.L. (2015). Handbook of clinical audiology. Philadelphia, PA: Lippincot, Williams & Wilkins.
Garbutt, S. & Harris, C. (1999). A review of optokinetic nystagmus (OKN) in infants and children. British Journal of Ophthalmology. 5(6), 1-10.
Valmaggia, C., Proudlock, F. & Gottlob, I. (2005). Look and stare optokinetic nystagmus in healthy subjects and in patients with no measureable binocularity: a prospective study. Klinische Monatsblätter für Augenheilkunde. 222(3), 196-201. View
Honrubia, V. (1968). Experimental studies on optokinetic nystagmus II. Normal Humans. Acta Oto-laryngologica. 65(1- 6), 441-448. View