Journal of Rehabilitation Practices and Research Volume 6 (2025), Article ID: JRPR-188
https://doi.org/10.33790/jrpr1100188Research Article
Feasibility and Preliminary Effects of Multichannel Functional Electrical Stimulation Plus Task-Specific Training on Upper-Extremity Outcomes During Inpatient Stroke Rehabilitation: A Pilot Randomized Study
Priya Karakkattil1*, PT, PhD, Caitlin Boyd2, MOT, OTR, Sofiya Mistry3, PT DPT, and Meredith Simone4, MOT, OTR
1School of Physical Therapy, Texas Woman’s University Institute of Health Sciences, 5500 Southwestern Medical Ave., Dallas, TX 75235, United States.
2College of Rehabilitative Sciences, University of St. Augustine for Health Sciences, United States.
3,4Baylor Institute for Rehabilitation, Frisco, Texas, United States.
Corresponding Author Details: Priya Karakkattil, PT, PhD, Assistant Professor, School of Physical Therapy, Texas Woman’s University Institute of Health Sciences, 5500 Southwestern Medical Ave., Dallas, TX 75235, United States.
Received date: 16th August, 2025
Accepted date: 24th October, 2025
Published date: 27th October, 2025
Citation: Karakkattil, P., Boyd, C., Mistry, S., & Simone, M., (2025). Feasibility and Preliminary Effects of Multichannel Functional Electrical Stimulation Plus Task-Specific Training on Upper-Extremity Outcomes During Inpatient Stroke Rehabilitation: A Pilot Randomized Study. J Rehab Pract Res, 6(2):188.
Copyright: ©2025, This is an open-access article distributed under the terms of the Creative Commons Attribution License 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective: To evaluate the feasibility and preliminary effects of a multichannel functional electrical stimulation (FES) combined with task-specific training on upper extremity outcomes during inpatient stroke rehabilitation.
Methods: In this two-group pretest–posttest randomized pilot study, individuals with unilateral hemiplegia post-stroke were randomized to either multichannel FES plus task-specific training (Xcite group, n=5) or traditional rehabilitation (n=5) alone over a two-week inpatient period. The intervention targeted coordinated activation of both proximal and distal upper limb muscles using a 12-channel FES device. Outcomes included shoulder active range of motion (AROM), grip strength, Action Research Arm Test (ARAT), Box and Block Test (BBT), Nine-Hole Peg Test (NHPT), and modified Patient-Specific Functional Scale (mPSFS).
Results: The Xcite (FES) group showed significantly greater between-group gains in shoulder flexion (p = 0.02, d = 1.48 [.02, 2.9]) and mPSFS (p = 0.04, d = 1.25 [-.16, 2.60]) compared with traditional rehabilitation. Within-group improvements in the FES group were significant for shoulder flexion (p = 0.02, d = -1.25, [-2.43, -.01]), ARAT (p = 0.03, d = -1.12 [-2.24,.07]), and mPSFS (p = 0.03, d = -1.59, [-3.10, .002]), whereas the traditional group improved only in mPSFS (p = 0.02, d = -1.38 [-2.09. -.08]). All FES participants completed the program within standard therapy time, with setup durations remaining manageable. Effects were moderate to large for shoulder flexion AROM, ARAT, mPSFS and smaller for grip strength and dexterity. The greatest gains observed in outcomes aligned with the intervention’s movement patterns.
Conclusion: Multichannel FES combined with task-specific training appears to be a feasible and promising intervention for improving upper extremity function during the early phase of stroke recovery. These preliminary findings highlight the potential benefit of this approach and provide guidance for optimizing intervention parameters and designing larger, controlled trials to confirm efficacy and establish standardized clinical protocols.
Keywords: Stroke rehabilitation, Functional Electrical Stimulation, Multichannel FES, Upper Extremity Function, Upper Extremity Mobility, Task-specific training
Introduction
Background and Purpose
Stroke is the fifth leading cause of death in the United States, and approximately 87% of stroke survivors report long-term disability that impacts participation in daily life [1]. Between 55% and 75% of individuals with stroke experience upper extremity (UE) impairments, resulting in difficulty performing activities of daily living [2]. Only 11.6% of survivors of ischemic middle cerebral artery stroke achieve full recovery of UE function within six months [3]. Recent advances in neurorehabilitation have emphasized task-specific, repetitive training to harness neuroplasticity for functional recovery [4]. Several rehabilitation strategies based on neuroplasticity principles have shown varying levels of evidence in improving UE function [5-7].
Functional electrical stimulation (FES) is a restorative intervention that activates the neuromuscular system through externally applied electrical currents to facilitate motor recovery and assist with motor function in individuals with neurological impairments [8]. Traditional FES systems are often limited to two channels, stimulating a single muscle group at a time. Cyclic FES delivers preprogrammed stimulation patterns for muscle re-education and endurance, while switch-triggered neuromuscular electrical stimulation (NMES) relies on external input to initiate stimulation, making it suitable for individuals with limited voluntary control [9]. Contralaterally controlled NMES activates the paretic limb based on movement in the unaffected limb, promoting bilateral cortical engagement [9]. EMG-triggered FES requires volitional effort to initiate stimulation, fostering active participation and neuroplasticity [9]. More advanced systems, such as those integrating brain-computer interfaces (BCIs) or sensor-based feedback, allow real-time modulation of stimulation based on user intent or biomechanics [9].
Recently, multichannel FES devices have emerged to provide stimulation patterns informed by the concept of muscle synergies, providing coordinated stimulation of select muscle groups in a naturally coordinated way [8]. The Xcite system is a multichannel FES device that offers 12-channel stimulation with preprogrammed sequences and audiovisual cues, enabling coordinated activation of select muscle groups in a manner that reflects natural movement patterns to support functional practice (Restorative Therapies, Inc., Baltimore, MD, USA). While clinically available, the impact of such coordinated stimulation on functional outcomes remains underexplored. Muscle synergies are believed to reflect the neurological organization of motor control, wherein groups of muscles are activated as functional units to simplify the coordination of complex movements [8]. Based on this concept, we hypothesize that coordinated multichannel stimulation of specific muscle groups during task-specific training will promote more natural movement patterns and facilitate upper limb recovery following stroke.
Though studies have shown that FES when used in conjunction with task-specific training, was effective in improving hand function, the duration of treatment and timing post-stroke varied significantly [5,10]. The majority of the improvement in upper extremity function post-stroke has been reported within 3 months after onset [11]. Yet, few studies have systematically evaluated the impact of FES during early phase of recovery, particularly within the context of inpatient rehabilitation, where the average length of stay is only 14.6 days [1].
Delivering coordinated multichannel stimulation during this critical window of spontaneous neurological recovery may enhance motor outcomes by reinforcing normal movement patterns through task specific neuroplastic adaptation. Therefore, assessing the efficacy of a multichannel FES during inpatient rehabilitation is essential to determine its suitability as an intervention within the constraints of this limited treatment window. To date, no studies have evaluated the efficacy and feasibility of a multichannel FES approach that delivers coordinated stimulation to specific muscle groups targeting both proximal and distal upper extremity muscles during the inpatient phase of post-stroke rehabilitation.
This pilot study aimed to explore the preliminary effects of a two week intervention using multichannel FES combined with task specific training during inpatient rehabilitation, compared to standard inpatient therapy alone, in individuals with unilateral hemiplegia following stroke. Specifically, the study evaluated changes in upper extremity mobility, function, and self-reported performance, and assessed the feasibility of implementing a 30-minute multichannel FES session within the constraints of routine inpatient therapy. The primary outcome measures were change scores in the Action Research Arm Test (ARAT), Box and Block Test (BBT), and Nine Hole Peg Test (NHPT).
Materials and Methods
Design: This experimental pilot study employed a two-group, pretest-posttest, randomized controlled design to explore the preliminary effects of a two-week intervention. Participants with unilateral hemiplegia following stroke were recruited through convenience sampling from an inpatient rehabilitation facility and then randomized to groups using a lot-drawing method without replacement of group allocation slips. This ensured equal allocation to the Xcite Group (multichannel FES + task-specific training) or the Traditional Rehabilitation Group (standard inpatient therapy). The randomization sequence was generated by an investigator (PK) who was not involved in intervention delivery or outcome assessment. Following informed consent and baseline assessment, one of the interventionalists drew a slip from an envelope to determine group allocation. The Institutional review board at Baylor Scott and White Research Institute approved this study (020-306). All participants provided written informed consent after receiving a detailed explanation of study procedures, potential benefits, and possible risks (e.g., mild skin irritation, transient muscle soreness, or fatigue). Safety screening excluded individuals with known contraindications to NMES. This study was prospectively registered with ClinicalTrials.gov (registration number: NCT04876703).
Participants
• Onset of stroke less than 3 months before enrollment.
• Unilateral upper extremity hemiparesis or hemiplegia
• Over the age of 18
Participants who had comorbidities impacting motor function (Parkinson's, congenital disorders) contraindications for NMES (seizure disorders, uncontrolled cardiac conditions, cancer, pacemaker, significant sensory deficits, contractures involving the shoulder, elbow, wrist, or finger joints, pregnancy, unhealed fractures), or a cerebellar stroke, and who were unable to follow two- step commands were excluded from the study. Inpatient rehabilitation therapists screened patients with unilateral stroke for eligibility, and informed suitable candidates about the study. Interested individuals were then contacted by an investigator who provided detailed information and answered questions. Participants were informed of their rights and asked to sign the approved informed consent.
Procedures
In this interdisciplinary research, two trained investigators (one physical therapist and one occupational therapist) performed all the outcome measures for all participants. The pretest measurements were taken within 96 hours before starting the research protocol. The testers were not blinded to group allocation. Before signing consent, participants underwent the Cognistat assessment to verify their cognitive capacity, per IRB requirements. The following demographic data were collected for each participant: age, gender, type and location of stroke, side affected, and time since onset. Pretest outcome measures were performed in the following order: Shoulder active range of motion (AROM), grip strength, Nine-Hole Peg Test (NHPT), Box and Block Test (BBT), Action Research Arm Test (ARAT), and Modified Patient-Specific Functional Scale (mPSFS). All measurements were tested on the unaffected side followed by the affected side. Upon completing the intervention, follow-up measurements were conducted within 24 hours by the same investigators, following the same order as previously described.
Intervention
The experimental group (Xcite) received functional electrical stimulation (FES) via the Xcite system for 30 minutes a day, 4 days a week, over 2 weeks, in conjunction with traditional rehabilitation for 5 days a week. Each FES session lasted 45 minutes, including setup, and was administered by the same trained physical therapist or occupational therapist. The Xcite system, which includes over 40 functional activities, was used to train participants in forward reach, grasp, and release tasks(“Restorative Therapies,” n.d.). Electrodes (2x2 cm to 2x3.5 cm) were placed on the rhomboid major, rhomboid minor, upper latissimus dorsi, anterior deltoid, posterior deltoid, triceps, extensor digitorum, flexor digitorum superficialis, and flexor pollicis longus on the affected UE. After electrode placement, each muscle group was individually stimulated following Xcite protocol to achieve optimal contractions. Stimulation parameters were individualized per participant to elicit optimal contractions and comfort, with pulse widths typically ranging from 200–300 μs, frequencies between 30–50 Hz, and amplitudes adjusted as needed (generally within 20–80 mA, depending on participant tolerance and muscle response). Participants completed multiple repetitions of reach-grasp-release tasks during each session. Objects that promoted an overhand cylindrical grasp such as foam blocks and balls were used during the task and were released into a bucket positioned at the starting point. Each session lasted 30 minutes and consisted of multiple sets of reach, grasp, release tasks. Each set included 8 to 12 repetitions, with rest intervals of 30–60 seconds between sets to minimize fatigue. The number of sets completed varied based on individual ability and tolerance. Audiovisual feedback from the Xcite system was combined with verbal and tactile cueing from the therapist to enhance engagement and task performance. The control group (Traditional Rehab) received the traditional rehabilitation at the therapist's discretion based on individual patient needs five days a week.
Outcome Measures
Shoulder active range of motion of flexion and abduction were assessed in supine using a universal goniometer. Two measurements were taken, and the average degree of measurement was recorded. For the assessment of shoulder ROM, the goniometer is a reliable and valid tool [12]. Grip strength was measured using a hand-held dynamometer (Jamar hydraulic hand dynamometer [Patterson Medical, Bolingbrook, IL]). Participants sat in a chair with the shoulder in neutral rotation and adduction, the elbow at 90 degrees of flexion and the forearm in a neutral position. A score for maximal grip was calculated by taking an average across three trials. Grip strength assessment using a hand-held dynamometer is a reliable test to assess maximal grip strength measurement in individuals with stroke within 3 months of onset [13].
The Nine-Hole Peg Test (NHPT) is an assessment of fine motor dexterity. The test includes the use of a stopwatch to calculate the amount of time needed to place and remove 9 pegs in holes on a pegboard. A score was calculated for the NHPT by the time taken to complete the activity in seconds. The average of two measurements of the NHPT were recorded. The NHPT is valid and reliable for assessing fine motor dexterity in individuals post-stroke [14,15]. The Box and Block Test (BBT) is a test to assess unilateral gross motor coordination of the UE. This assessment measures the number of blocks a participant could grasp and transfer from one compartment to the next in one minute. The BBT score was determined by counting the number of blocks transferred, with the average of two measurements recorded. The BBT has been reported as a valid and reliable tool for assessing gross motor coordination [14,15]. The Action Research Arm Test is a 19-item measure with 4 sub categories (grasp, grip, pinch, and gross arm movement) to assess the UE function. Each item on the ARAT is scored on a 4-point Likert Scale with the total score ranging from 0-57, with a higher score indicating better performance. ARAT is valid and reliable when assessing overall upper extremity function in individuals post-stroke [15,16]. Participant’s perception of improvement in upper extremity function was assessed using a modified Patient-Specific Functional Scale (mPSFS). PSFS is a subjective assessment of a patient's ability to complete self-identified activities that are important to the patient. The PSFS was scored on an 11-point scale used to rate the ability to complete tasks, with a score of '0' being "Unable to perform" and a score of 10 being "Able to perform at prior level." For our study, we used a modified version of the PSFS by asking the participant to rate their current upper extremity function. The PSFS has been recommended as a reliable tool to use during subacute stroke rehabilitation [17].
These measures were selected to capture a spectrum of upper extremity outcomes relevant to inpatient stroke rehabilitation, including joint mobility (AROM), strength (grip), gross and fine dexterity (BBT, NHPT), functional performance (ARAT), and patient-perceived function (mPSFS). ARAT was chosen over other options due to its combined assessment of gross and fine motor skills and its established responsiveness in early stroke rehabilitation.
Statistical Analysis
All statistical analyses were conducted using IBM SPSS Statistics for Windows, Version 28. Descriptive statistics (means and standard deviations) were calculated for demographic characteristics and all dependent variables at pre-test, post-test, and for change scores. Although the primary purpose of this pilot study was to explore preliminary effects and estimate effect sizes, inferential tests were also conducted with caution, given the small sample size. Normality of the data was assessed using Shapiro-Wilk tests and visual inspection of histograms and Q-Q plots. While some deviations from normality were observed in a few variables, the t-tests were retained for analysis, as they are considered robust to moderate violations of normality, particularly with equal group sizes and similar variances. Group differences at baseline for continuous demographic and outcome variables were examined using one-tailed independent t-tests. For variables that violated the assumption of homogeneity of variance, corrected p-values (Welch’s t-test) were reported. Between group differences from pre- to post-test were analyzed using change scores. Levene’s test confirmed homogeneity of variance for the change scores, supporting the use of one-tailed independent t-tests, given that the a priori hypothesis specified directional improvement. Within-group changes were evaluated using one-tailed paired t-tests. Effect sizes (Cohen’s d) were calculated for between and within group comparisons to estimate the magnitude of the observed effects. An alpha level of .05 was used for all inferential tests; however, given the number of outcome measures examined, no adjustments were made for multiplicity. Accordingly, all findings should be interpreted as exploratory, with emphasis placed on effect size estimation rather than statistical significance due to the pilot nature of this study.
Results
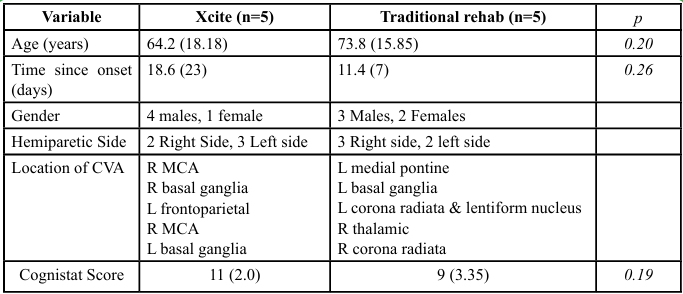
Ten participants completed the study, with five randomized to the experimental group (Xcite) and five to the control group (Traditional Rehabilitation). Table 1 presents descriptive statistics for participant demographics. Demographic characteristics were similar between the two groups. Given the small sample size, we focused on reporting descriptive statistics and estimating effect sizes to inform the design and sample size calculations of future larger-scale trials.
Between-Group Differences in Change Scores
Descriptive statistics, change scores, p values and between-group effect sizes (Cohen’s d) for all outcome measures are presented inTable 2. Independent-samples t tests were conducted for completeness; however, the primary emphasis was placed on effect sizes due to the pilot nature and small sample size. For the between group change scores, large effect sizes favoring the Xcite group were observed for shoulder flexion active range of motion (d = 1.48) and the modified Patient-Specific Functional Scale (d = 1.25). Moderate effects were found for the Action Research Arm Test (d = 0.70) and shoulder abduction AROM (d = 0.59). Smaller between-group effects were noted for grip strength (d = 0.38), and the Box and Block Test (d = 0.06).
Because several participants were unable to perform the Nine-Hole Peg Test (NHPT) at baseline and post-intervention, the outcome was coded as a dichotomous variable (able vs. unable to perform). Chi-square tests of independence were conducted to examine the association between group and NHPT performance status at both time points. Due to small expected cell counts, Fisher’s Exact Test was used. There was no significant association between group and NHPT performance at baseline (p = 0.78), Cramer’s V <.001 or post intervention (p = 0.50), Cramer’s V>.218.
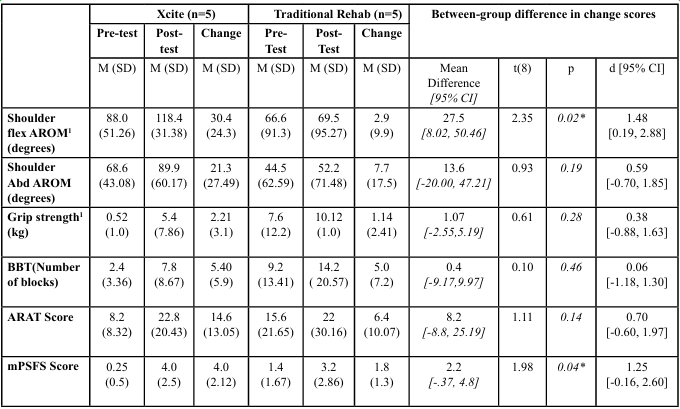
Table 2: Descriptive Statistics of Pre-test, Post-test, and Change Scores of Dependent Variables on Affected UE, and Findings from Independent t-test for Change Scores
Within-Group Changes
Table 3 summarizes the within-group paired t test results and corresponding effect sizes (Cohen’s d). While some improvements have reached statistical significance, emphasis is placed on effect sizes to illustrate the magnitude of change within each group.
The Xcite group demonstrated large effect sizes for shoulder flexion active range of motion (d = 1.25) and the Action Research Arm Test (d = 1.12), compared to small to moderate effects in the control group (d = 0.29 and 0.64, respectively). Both groups showed large effects on the modified Patient-Specific Functional Scale (Xcite: d = 1.59; Control: d = 1.38). For shoulder abduction AROM, the Xcite group demonstrated a notably larger within-group improvement, with a large effect size (d = 0.85), compared to a smaller effect observed in the Traditional Rehabilitation group (d = 0.44).
Box and Block Test (BBT) scores in the Xcite group approached a large effect size (d = 0.92), despite limited participant completion, whereas the control group showed a medium effect (d = 0.70). Grip strength changes reflected a medium effect in the Xcite group (d = 0.71) and a small effect in the control group (d = 0.48). These findings highlight meaningful within-group improvements in the Xcite group, with several outcomes showing moderate to large effects despite the small sample size.
Completion of Outcome Measures
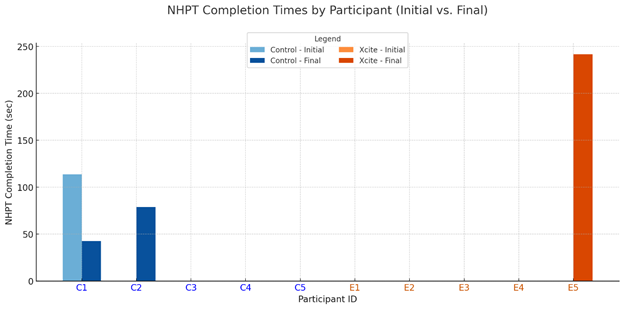
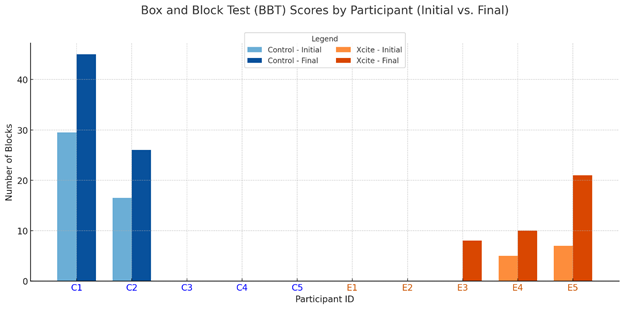
At pre-test, only one participant in the Traditional Rehabilitation group and none in the Xcite group were able to complete the Nine- Hole Peg Test (NHPT). Post-intervention, two participants in the Traditional group and one in the Xcite group completed the test (see Figure 1). For the Box and Block Test (BBT), two participants from each group completed the assessment at pre-test. Following the intervention, three participants in the Xcite group were able to complete the BBT, while the number remained the same in the Traditional group (see Figure 2). Regarding the Action Research Arm Test (ARAT), three participants in the Xcite group and two in the Traditional group completed the test at pre-test. Post-intervention, the number of completers remained unchanged in the Traditional group, while one additional participant in the Xcite group was able to complete the ARAT (see Figure 3).
Feasibility
Feasibility was operationalized as the ability to deliver all planned sessions within routine therapy time without missed appointments or scheduling conflicts. All five participants in the experimental group completed the eight planned FES sessions with 100% attendance and zero missed appointments. Setup and preparation averaged less than 15 minutes per session, allowing delivery of the full 30-minute FES treatment within the 45-minute allocated timeframe. No adverse events were reported. These findings indicate that the multichannel FES intervention was feasible to implement within the constraints of routine inpatient rehabilitation without requiring schedule adjustments.
Discussion
To the best of our knowledge, this study is the first to evaluate the preliminary effects of coordinated stimulation using a multichannel functional electrical stimulation (FES) system targeting both proximal and distal muscles in conjunction with task-specific activity to improve upper extremity mobility and function in individuals with stroke during inpatient rehabilitation. The findings from this pilot study suggest that a multichannel FES system, such as Xcite, may be a promising intervention for restoring upper extremity function during early stroke rehabilitation in an inpatient setting. However, these findings are preliminary and are intended to inform the design and implementation of future larger-scale clinical trials.
Shoulder flexion active range of motion (AROM) improved more in the Xcite group than in the traditional rehabilitation group, with a large between-group effect size. This improvement likely reflects the specificity of the forward reach task, which emphasizes shoulder flexion. In contrast, the change in the traditional group did not exceed the minimum detectable change (MDC) of 5 degrees. Although shoulder abduction AROM improved in both groups, the Xcite group achieved three times greater gains, with a moderate effect size. The smaller change in abduction may be due to the task targeting flexion more than abduction. These findings underscore the potential of multichannel FES combined with task-specific training to enhance shoulder mobility during early stroke rehabilitation. They are consistent with previous research showing joint-specific gains such as improved finger motion when FES is applied to targeted muscles [18], highlighting the importance of task and stimulation specificity in promoting motor recovery.
Smaller between-group effects were observed for grip strength, with a Cohen’s d of 0.38, indicating a small effect size. Although the Xcite group showed greater within-group gains than the traditional group, neither reached the smallest real change (SRC) of 2.9 kg reported by Bertrand et al. [13]. While the protocol included a grasp-and-release task, its intensity and specificity may have been insufficient to drive meaningful grip strength improvements. According to neuroplasticity principles, particularly specificity and intensity, future studies should incorporate more grip-focused FES activities. Hara et al. [19] reported significant grip strength gains after five months of EMG-triggered FES in chronic stroke, suggesting that longer intervention durations may also be needed to achieve clinically meaningful outcomes for grip strength [19].
Smaller between-group effects were found for the Box and Block Test (BBT; d = 0.06), indicating limited differential impact on dexterity. However, more participants in the Xcite group were able to complete the BBT post-intervention, and a large within-group effect size supports potential clinical relevance. The grasp-and-release task likely aligned more closely with the BBT’s motor demands, contributing to functional gains. In contrast, the NHPT’s fine motor requirements were not directly addressed by the intervention. These findings align with Alon et al. [2], who similarly found no significant differences in BBT outcomes between FES and control groups. Overall, the results highlight the importance of task specificity when designing FES interventions to target dexterity in stroke rehabilitation.
Upper extremity function, measured by the Action Research Arm Test (ARAT), showed a moderate to large between-group effect size, suggesting a clinically meaningful benefit for the Xcite group over traditional rehabilitation. Importantly, the mean ARAT score improvement in the Xcite group was within the reported minimal clinically important difference (MCID) of 12 -17 points for individuals during the early stage of stroke [20], indicating that the observed change is likely to be meaningful in everyday functional activities. The ARAT assesses both gross motor function and dexterity [21], and the repeated reaching and grasping tasks in the Xcite protocol likely contributed to improvements in gross upper limb movement. While Yang et al. [10] demonstrated the effectiveness of FES for ARAT outcomes in chronic stroke, this pilot study extends the evidence to the inpatient (subacute) phase, supporting the early use of multichannel FES. These findings underscore the value of task- specific, functionally relevant interventions early in stroke recovery to optimize upper limb outcomes.
Perceived upper extremity function, measured by the modified Patient-Specific Functional Scale (mPSFS), improved meaningfully in both groups, with changes exceeding the minimal important change of 1.58 points for individuals with stroke [17]. A large between-group effect size favoring the Xcite group suggests greater perceived benefit from multichannel FES. As self-perceived function reflects the individual’s experience and informs shared decision- making, these results underscore the importance of integrating patient-reported outcomes in stroke rehabilitation research [22]. While advanced rehabilitation technologies often aim for increased precision, their clinical success depends on patient acceptance a balance between usability and perceived benefit. The positive reports from participants, particularly in the Xcite group, indicate that the intervention was both effective and acceptable within the inpatient rehabilitation setting.
Enhanced upper extremity performance observed in the FES group may be attributed to several neurophysiological mechanisms. Functional electrical stimulation (FES) activates both motor and sensory pathways, providing afferent input to the central nervous system that is essential for motor learning and cortical reorganization [23]. This sensory input may drive neuroplasticity by enhancing sensorimotor integration and promoting reorganization within motor-related cortical areas [24]. Repeated task-specific FES may reinforce residual corticospinal pathways and improve voluntary motor control. Importantly, the multichannel FES used in this study provided coordinated stimulation across both proximal and distal upper limb muscles, aligning with the concept of muscle synergies— functionally organized patterns of muscle activation that underlie natural movement [8]. This coordinated stimulation may further enhance neuroplasticity by engaging distributed motor networks in a way that mirrors typical motor control strategies. Additionally, the concurrent activation of volitional movement and peripheral stimulation may increase motor cortex excitability more effectively than voluntary movement alone [25]. Functional imaging studies have shown that FES can induce activity changes in sensorimotor areas and support reorganization following stroke [26]. When paired with goal-directed, meaningful activities as in this study coordinated multichannel FES may optimize both peripheral and central mechanisms of motor recovery, contributing to the observed improvements.
The successful integration of multichannel FES with task-specific activities into routine inpatient therapy sessions supports the feasibility of implementing this intervention during stroke rehabilitation. All participants in the FES group were able to complete the scheduled 30-minute sessions within their assigned rehabilitation time, indicating compatibility with typical clinical workflows. The average setup time was less than 15 minutes. This duration aligned with the clinical time limits of the inpatient care structure and did not interfere with the participants' scheduled therapy time. Importantly, the FES group demonstrated greater improvements in shoulder mobility and upper extremity function than the traditional rehabilitation group, with larger effect sizes supporting the potential clinical value. These findings suggest that multichannel FES is both feasible and promising as an early-phase intervention to enhance functional recovery within the limited timeframe of inpatient rehabilitation.
Limitations
We acknowledge that the small sample size limits the ability to draw definitive conclusions or demonstrate statistical significance within and between groups. However, as a pilot study conducted during inpatient rehabilitation, this investigation provides valuable preliminary data on the use of multichannel FES combined with task- specific training. The effect sizes observed across multiple outcome measures offer important insights into potential clinical benefits and will inform the design and sample size calculations for future, larger-scale studies. In addition to increasing the sample size, future research should consider incorporating a broader range of task- specific activities beyond forward grasp and release to better target and evaluate improvements in dexterity. Outcome assessors were not blinded to group allocation, which may have introduced detection bias in clinical outcome measures. Future efficacy trials should incorporate blinded outcome assessment to minimize potential bias in measuring treatment effects. Additional limitations include the short intervention duration, which may not fully reflect the potential for long-term functional gains, and the lack of formal assessment of patient or therapist perspectives related to feasibility. Setup time was based on therapist estimates rather than systematic measurement, which may affect the accuracy of reported durations.
Clinical Implications
This pilot study suggests that multichannel functional electrical stimulation (FES) combined with task-specific training is both feasible and potentially effective during the inpatient (subacute) phase of stroke rehabilitation. Clinically meaningful improvements in shoulder mobility, upper extremity function, and patient-reported outcomes were observed, with moderate to large effect sizes favoring the FES group. These findings support the integration of multichannel FES into routine rehabilitation to enhance upper limb recovery. The inclusion of patient-centered measures, such as the mPSFS, underscores the value of incorporating the individual’s perspective when tailoring interventions. Clinicians should prioritize task specificity, appropriate intensity, and patient acceptance when designing FES protocols. Building on these promising results, future larger-scale studies are encouraged to refine standardized protocols and explore the broader applicability of multichannel FES across diverse clinical settings. Based on the effect sizes observed in this pilot, preliminary sample size estimates can guide the design of adequately powered trials to confirm these early findings.
Conflicts of Interest:
The authors have no conflicts of interest to disclose regarding the choice or use of the Xcite FES.
Acknowledgements:
We would like to thank David Lo, MD and Kelly Beck, PhD at Baylor Scott and White Institute for Rehabilitation for their guidance during the recruitment and data collection of this research study.
We would also like to thank Ryan Hulla, PhD, the statistician at Texas Woman’s University, for his guidance with statistical analysis.
This study was funded in part by the Baylor Scott and White Health Care System Foundation Grant, which supported the purchase of equipment used in this research.
References
Tsao, C. W., Aday, A. W., Almarzooq, Z. I., Anderson, C. A. M., Arora, P., Avery, C. L., Baker-Smith, C. M., Beaton, A. Z., Boehme, A. K., Buxton, A. E., Commodore-Mensah, Y., Elkind, M. S. V., Evenson, K. R., Eze-Nliam, C., Fugar, S., Generoso, G., Heard, D. G., Hiremath, S., Ho, J. E., Kalani, R., Kazi, D. S., Ko, D., Levine, D. A., Liu, J., Ma, J., Magnani, J. W., Michos, E. D., Mussolino, M. E., Navaneethan, S. D., Parikh, N. I., Poudel, R., Rezk-Hanna, M., Roth, G. A., Shah, N. S., St-Onge, M.-P., Thacker, E. L., Virani, S. S., Voeks, J. H., Wang, N.-Y., Wong, N. D., Wong, S. S., Yaffe, K., Martin, S. S., et al. (2023). Heart Disease and Stroke Statistics — 2023 Update: A Report from the American Heart Association. Circulation, 147(8), e93–e621. View
Alon, G., Levitt, A. F., & McCarthy, P. A. (2007). Functional electrical stimulation enhancement of upper extremity functional recovery during stroke rehabilitation: A pilot study. Neurorehabilitation and Neural Repair, 21(3), 207–215. View
Kwakkel, G., Kollen, B. J., van der Grond, J., & Prevo, A. J. H. (2003). Probability of regaining dexterity in the flaccid upper limb: Impact of severity of paresis and time since onset in acute stroke. Stroke, 34(9), 2181–2186. View
Kleim, J. A., & Jones, T. A. (2008). Principles of experience dependent neural plasticity: Implications for rehabilitation after brain damage. Journal of Speech, Language, and Hearing Research: JSLHR, 51(1), S225-239. View
Eraifej, J., Clark, W., France, B., Desando, S., & Moore, D. (2017). Effectiveness of upper limb functional electrical stimulation after stroke for the improvement of activities of daily living and motor function: A systematic review and meta analysis. Systematic Reviews, 6(1), 40. View
Nogueira, N. G. de H. M., Parma, J. O., Leão, S. E. S. de A., Sales, I. de S., Macedo, L. C., Galvão, A. C. D. R., de Oliveira, D. C., Murça, T. M., Fernandes, L. A., Junqueira, C., Lage, G. M., & Ferreira, B. de P. (2021). Mirror therapy in upper limb motor recovery and activities of daily living, and its neural correlates in stroke individuals: A systematic review and meta analysis. Brain Research Bulletin, 177, 217–238. View
Wu, J., Zeng, A., Chen, Z., Wei, Y., Huang, K., Chen, J., & Ren, Z. (2021). Effects of Virtual Reality Training on Upper Limb Function and Balance in Stroke Patients: Systematic Review and Meta-Meta-Analysis. Journal of Medical Internet Research, 23(10), e31051. View
Cheung, V. C. K., Niu, C. M., Li, S., Xie, Q., & Lan, N. (2019). A Novel FES Strategy for Poststroke Rehabilitation Based on the Natural Organization of Neuromuscular Control. IEEE Reviews in Biomedical Engineering, 12, 154–167. View
Knutson, J. S., Fu, M. J., Sheffler, L. R., & Chae, J. (2015). Neuromuscular Electrical Stimulation for Motor Restoration in Hemiplegia. Physical Medicine and Rehabilitation Clinics of North America, 26(4), 729–745. View
Yang, J.-D., Liao, C.-D., Huang, S.-W., Tam, K.-W., Liou, T.-H., Lee, Y.-H., Lin, C.-Y., & Chen, H.-C. (2019). Effectiveness of electrical stimulation therapy in improving arm function after stroke: A systematic review and a meta-analysis of randomised controlled trials. Clinical Rehabilitation, 33(8), 1286–1297. View
Nakayama, H., Jørgensen, H. S., Raaschou, H. O., & Olsen, T. S. (1994). Recovery of upper extremity function in stroke patients: The Copenhagen Stroke Study. Archives of Physical Medicine and Rehabilitation, 75(4), 394–398. View
Gajdosik, R. L., & Bohannon, R. W. (1987). Clinical measurement of range of motion. Review of goniometry emphasizing reliability and validity. Physical Therapy, 67(12), 1867–1872. View
Bertrand, A. M., Fournier, K., Wick Brasey, M.-G., Kaiser, M.-L., Frischknecht, R., & Diserens, K. (2015). Reliability of maximal grip strength measurements and grip strength recovery following a stroke. Journal of Hand Therapy: Official Journal of the American Society of Hand Therapists, 28(4), 356–362; quiz 363. View
Chen, H.-M., Chen, C. C., Hsueh, I.-P., Huang, S.-L., & Hsieh, C.-L. (2009). Test-retest reproducibility and smallest real difference of 5 hand function tests in patients with stroke. Neurorehabilitation and Neural Repair, 23(5), 435–440. View
Lin, K., Chuang, L., Wu, C., Hsieh, Y., & Chang, W. (2010). Responsiveness and validity of three dexterous function measures in stroke rehabilitation. Journal of Rehabilitation Research and Development, 47(6), 563–571. View
Yozbatiran, N., Der-Yeghiaian, L., & Cramer, S. C. (2008). A standardized approach to performing the action research arm test. Neurorehabilitation and Neural Repair, 22(1), 78–90. View
Evensen, J., Soberg, H. L., Sveen, U., Hestad, K. A., Moore, J. L., & Bronken, B. A. (2023). Measurement Properties of the Patient-Specific Functional Scale in Rehabilitation for Patients With Stroke: A Prospective Observational Study. Physical Therapy, 103(5), pzad014. View
Gharib, N. M. M., Aboumousa, A. M., Elowishy, A. A., Rezk Allah, S. S., & Yousef, F. S. (2015). Efficacy of electrical stimulation as an adjunct to repetitive task practice therapy on skilled hand performance in hemiparetic stroke patients: A randomized controlled trial. Clinical Rehabilitation, 29(4), 355–364. View
Hara, Y., Obayashi, S., Tsujiuchi, K., & Muraoka, Y. (2013). The effects of electromyography-controlled functional electrical stimulation on upper extremity function and cortical perfusion in stroke patients. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 124(10), 2008–2015. View
Lang, C. E., Edwards, D. F., Birkenmeier, R. L., & Dromerick, A. W. (2008). Estimating minimal clinically important differences of upper-extremity measures early after stroke. Archives of Physical Medicine and Rehabilitation, 89(9), 1693–1700. View
Hsieh, Y., Wu, C., Lin, K., Chang, Y., Chen, C., & Liu, J. (2009). Responsiveness and Validity of Three Outcome Measures of Motor Function After Stroke Rehabilitation. Stroke, 40(4), 1386–1391. View
Reeves, M., Lisabeth, L., Williams, L., Katzan, I., Kapral, M., Deutsch, A., & Prvu-Bettger, J. (2018). Patient-reported outcome measures (PROMs) for acute stroke: Rationale, methods and future directions. Stroke, 49(6), 1549–1556. View
Chipchase, L. S., Schabrun, S. M., & Hodges, P. W. (2011). Peripheral electrical stimulation to induce cortical plasticity: A systematic review of stimulus parameters. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 122(3), 456–463. View
Stefan, K., Kunesch, E., Cohen, L. G., Benecke, R., & Classen, J. (2000). Induction of plasticity in the human motor cortex by paired associative stimulation. Brain, 123(3), 572–584. View
Khaslavskaia, S., & Sinkjaer, T. (2005). Motor cortex excitability following repetitive electrical stimulation of the common peroneal nerve depends on the voluntary drive. Experimental Brain Research, 162(4), 497–502. View
Wei, W., Bai, L., Wang, J., Dai, R., Tong, R. K., Zhang, Y., Song, Z., Jiang, W., Shi, C., Li, M., Ai, L., & Tian, J. (2013). A longitudinal study of hand motor recovery after sub-acute stroke: A study combined FMRI with diffusion tensor imaging. PloS One, 8(5), e64154. View