Journal of CAM Research Progress Volume 3 (2024), Article ID: JCRP-115
https://doi.org/10.33790/jcrp1100115Review Article
Assessing Kombucha: A Systematic Review of Health Effects in Human
Carlos Aulesa1*, Carmen Góngora2
1Emerit Biochemistry Professor, University Hospital Vall d'Hebron, Barcelona, Spain.
2Educacional phychologist, Independent consultand researcher Barcelona, Spain.
Corresponding Author: Carlos Aulesa, Emerit Biochemistry Professor, University Hospital Vall d'Hebron, Barcelona, Spain.
Received date: 28th November, 2023
Accepted date: 12th January, 2024
Published date: 15th January, 2024
Citation: Aulesa, C., & Góngora, C., (2024). Assessing Kombucha: A Systematic Review of Health Effects in Human. J CAM Res Progress, 3(1): 115.
Copyright: ©2024, This is an open-access article distributed under the terms of the Creative Commons Attribution License 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Abstract
Objective: To assess the new clinical evidence regarding the efficacy and safety of Kombucha for human health.
Background: Kombucha is one of the most popular and rapidly expanding functional beverages globally. It has gathered significant attention, primarily due to its claimed health benefits, such as enhancing the immune system and potentially possessing antidiabetic properties.
Methodology: Systematic review was performed following PRISMA 2020 guidelines without a meta-analysis. The aim was to analyze recent literature (within the past six years) on health publications and examine the trails of human benefits on kombucha consumption. Both authors independently conducted a comprehensive review and reached a conclusion. Tools were used to calculate Kappa agreement index between authors and create a PRISMA flowchart to assess study quality.
Results: Five significant bibliometric reviews and four new human trials were identified. The initial trial examining the impact of Kombucha on intestinal microbiota showed neutral/negative results in healthy individuals. However, in a second microbiota trial, Kombucha significantly improved symptoms among patients with irritable bowel syndrome. Moreover, two recent clinical trials on diabetes strongly suggest that Kombucha enhances carbohydrate metabolism, indicating a potential antidiabetic effect for diabetic individuals. Nevertheless, it is important to note that these results should be considered a positive preliminary pilot assay due to the limited number of patients.
Conclusions: Our research has shown that there is still controversy over the health benefits of Kombucha. Although there have been some limited clinical trials, the results are often contradictory, making it difficult to determine the positive effects of Kombucha on human health. The purpose of this review is to assess the conflicting information on the health benefits of consuming Kombucha.
Keywords: A systematic review, Kombucha Fermented Tea Everage, Camellia Sinensis, Nutraceutical Beverage, Functional Drink.
Introduction
Kombucha is a mildly acidic and carbonated beverage that results from fermenting specific types of tea sweetened with a symbiotic culture of bacteria and yeast (SCOBYs) [1,2]. It is a low-alcohol, fermented drink that has recently gained popularity and has significantly impacted the functional beverage market [3,4].
Kombucha contains various bioactive substances derived from the plant substrates used (such as tea, herbal extracts, or fruit juices) and from the metabolic activity of acetic acid, lactic acid bacteria and yeasts [2]. These bioactive compounds include phenolic compounds (such as catechins, theaflavins, and flavanols), organic acids (such as gluconic and glucuronic acids, as well as lactic acid), vitamins, minerals, and essential amino acids. Different groups of microorganisms, each with different concentrations, contribute to the production of these bioactive compounds [5].
As a result, Kombucha can be considered a positive source of probiotic substances in the food realm or a postbiotic functional beverage [6].
In addition to its functional properties, Kombucha has been documented to have other beneficial and therapeutic effects, including its role as an antioxidant, anti-inflammatory agent, anti aging solution, and potential anti-tumor activity. At the same time, some authors have periodically published articles conducting systematic reviews of clinical evidence related to Kombucha in recent years [10-16]. Nevertheless, some research argues that there is a lack of evidence from controlled clinical trials in humans to support these claimed health benefits [13,14].
In summary, this article aims to perform a contemporary bibliometric and systematic review of the potential health benefits of Kombucha as reported in the scientific literature. We will extensively search medical databases such as PubMed, WebScience, Clinical Trials.gov, Cochrane, Bireme, and Lilacs, focusing on the most recent six years.
Methods
Bibliographic Review
We conducted a systematic review, excluding statistical meta-analysis, following the 2020 Prisma Guide (http://www. prismstatement.org) [17]. An intense search of scientific literature and medical databases was conducted in English and Spanish. These databases included PubMed, WebScience, Cochrane, Bireme, Lilacs, and, lastly, Clinical Trials. gov, the clinical trials register of the National Library of Medicine (NLM). The comprehensive review was independently conducted by two authors of this work, one as an experienced researcher (C.A) and the other as a researcher in training (C.G). They established a protocol for selecting and excluding relevant publications. We standardized computer-based supports with various sections (title, authors, journal, year, abstract). The research strategy encompassed all studies aiming to develop associations related to specific terms or keywords used in the query.The MeSH (Medical Subject Headings) descriptors were searched for the terms "kombucha*," "kombucha tea," "kombucha beverage," "fermented kombucha beverage," "Camellia sinensis," "kombucha nutraceutical," "kombucha probiotic," "kombucha prebiotic," and "kombucha's health effects" in the context of” human beings”. The search did not specify a particular population. The article selection period was unrestricted and included publications until the paper submission date, primarily focusing on 2018 to 2023. All references were managed using the Mendeley reference management software. Regular meetings were held between the two researchers after completing the search on each platform (PubMed, WebScience, etc.) to establish effective feedback for training and to compare the findings of both authors while assessing the concordance of different options. Various quality control mechanisms were selected for the bibliographic review to evaluate internal validity (the robustness of the research and the absence of systematic errors) and external validity (by analyzing selection bias, detection performance, and loss of reporting). Some tools were developed to assess the quality of the studies, such as calculating the Kappa concordance index between the two authors during the selection of systematic reviews and clinical trials. Finally, a Prisma flowchart (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) was used to ensure the reliability of the results.
Exclusion and inclusion criteria of the review
The exclusion criteria involved experimental work with animals and in vitro tests with cultured cells, including human cells like fibroblasts and intestinal tumor cells (Caco-2 colorectal cancer cells), which were not the target of this review. The inclusion criteria for selected articles in the systematic review spanned the period from 2018 to 2023 to explore potential human health benefits and examine clinical evidence of biological activities related to Kombucha, along with advancements in health benefits associated with kombucha consumption.
Statistical analysis
The statistical analysis was performed using the Stata v.14.0 calculation program (STATA Corp, College Station, TX, USA). Cohen's Kappa agreement coefficient was calculated to assess the agreement between the two authors in selecting the articles resulting from the screening. The Kappa coefficient was interpreted using Bryt criteria (1966) as follows: Kappa <= 0 indicates no agreement, k = 0.01-0.20 signifies poor agreement, k = 0.21-0.40 indicates slight agreement, k = 0.41-0.60 reflects fair agreement, k = 0.61-0.80 indicates good agreement, k = 0.81 to 0.92 signifies good excellent agreement and kappa 0.93-1.0 denotes excellent agreement. Additionally, kappa indices adjusted for bias (BAK) and prevalence and bias (PARAB) were calculated [18- 21].Results
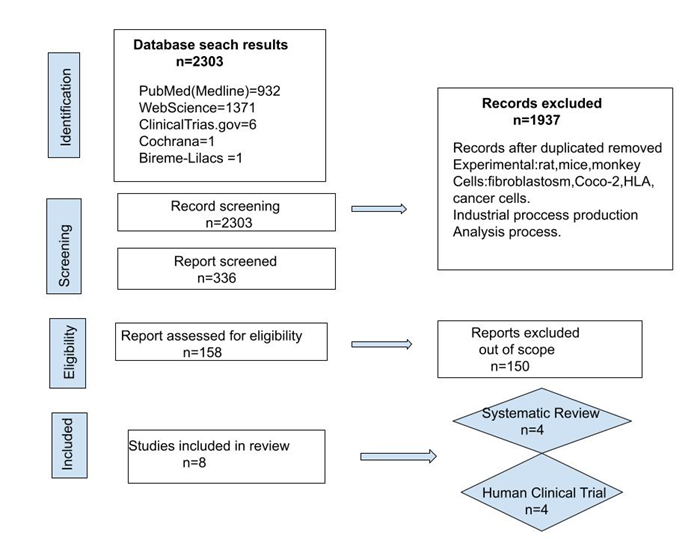
The primary objective of this work is to discover new information regarding the potential health effects on humans resulting from the consumption of Kombucha, following the inclusion and exclusion criteria outlined in the methods section. The results of our study selection process are presented in Flowchart (Figure 1). We initially identified 2303 articles in our electronic search, with 932 from PubMed using the keyword algorithm outlined in (Table 1) and 1371 from WebScience using the keyword algorithm described in (Table 1). Furthermore, we explored ClinicalTrials.gov and were left with three clinically relevant trials for eligibility. Analyzing the Cochrane database led us to a new trial related to diabetes, which is also appropriate for eligibility. Our exploration of the Bireme and Lilacs platforms in English and Spanish yielded no clinical trials.
In summary, out of the initial 2303 articles, 185 were excluded as duplicates, and 1989 were discarded due to their experimental nature involving no human being, such as rats, mice, monkeys, and so on. Some were in vitro tests with human cells, such as fibroblasts and intestinal tumor cells and others were related to industrial, chemical, and microbiological processes involved in kombucha production; all of them were discarded, which is not the target of this Review (Figure 1). Afterward, 129 potentially eligible studies were selected for further screening. The Kappa agreement index has been calculated between the two researchers for the 129 articles resulting from screening, and the agreement index for the 16 eligibility (C.A.) reports for (C.G.) is as follows:
Kappa 0.2372 (95% CI 0.0647-0.4097), Adjusted kappa for bias (BAK) k=0.2037, and Adjusted kappa for prevalence and bias (PARAB) k=0.4729, Agreement 73.6%. According to Byrt's scale [20,21], our results present a low rating that could be improved with experience in subsequent systematic review work. Following detailed evaluations, 16 articles were deemed suitable. Moreover, upon closer examination, seven reports were excluded as they fell outside the scope of the study. Finally, nine articles were included in the systematic review. The general characteristics of these five review-included studies are presented with a summary of the article in Table 2 and the description of four new clinical trials outlined in Table 3 and Table 4. In addition, the general characteristics of the four clinical trial studies included are presented in Table 5.
Discussion
Five literature reviews have been chosen following the inclusion criteria established in the method section. As such, we've opted for the reviews by Kapp [13] (2019) and Krieger [15] (2021), which propose classical systematic reviews to identify new publications discussing the efficacy of consuming Kombucha from a human health perspective. However, they highlight the need for more scientific literature in human clinical trials. Additionally, Morales [14] and Krieger [15] suggest that the probable cause is the microbial diversity of SCOBY, emphasizing the need to isolate and identify substances with therapeutic effects for reliable clinical trials. Furthermore, Esatbeyoglu [16] points out that the inherent complexity and lengthy process of industrial kombucha production and the need for more standardization of this beverage are the primary obstacles hindering clinical assays. This complexity and the absence of a universally accepted legal framework regulating industrial kombucha production in various countries compounds the challenge. In conclusion, the reviews by Martinez [12], Kapp [13], Morales [14], Krieger [15], and Esatbeyoglu [16] underscore the necessity for positive outcomes in human studies and highlight the insufficient scientific evidence supporting regular consumption of Kombucha.In her essay, Hanna Bergtrom from Sweden aimed to investigate the effect of Kombucha on the human gut microbiota through a clinical trial. The study focused on understanding whether Kombucha, which contains living microorganisms, has probiotic potential and how it impacts the body. The trial involved 42 healthy individuals aged 18 or older who were randomly assigned to drink either live or sterilized Kombucha (330 ml daily) or water (control) for three weeks. The bacterial characterization of fecal samples was analyzed by PCR Sanger sequencing, and the proposed statistical analyses involved ANOVA and Cluster plots. The study of the data shows a conclusive result: there is no effect from either live or sterilized Kombucha on the intestinal microbiota. Neither the clustering, the statistical analysis, nor the table of differences show a difference due to treatment. In brief, with a correct number of samples and employing robust statistics, the study found no effect of Kombucha on the intestinal flora after three weeks of consumption. Even though it is not statistically verifiable, several participants (three out of eight) observed positive changes during the study (improved consistency of the feces/ easier defecation), so other unknown health benefits of Kombucha remain in the microbiological community of the patient's gut.
Concerning the clinical assay of Pilipenko, V. et al. (2022) [23]. This trial assessed the short-term safety and tolerability of a new pasteurized, non-alcoholic beverage based on Kombucha enriched with inulin. The study included 40 participants with constipation predominant irritable bowel syndrome, randomly assigned to drink either 220 ml of the kombucha-based beverage with inulin or 220 ml of water (control group) for ten days. Participants completed surveys before and after the trial, focusing on variables like stool frequency, form, and accompanying symptoms. The student's t-test was used for statistical analysis to determine differences between the treatment and placebo groups. Results indicate that Kombucha is well tolerated and may improve both stool frequency and consistency in individuals with constipation-predominant irritable bowel syndrome. However, a more in-depth analysis of the clinical trial reveals that the pasteurized Kombucha used in the study differs from the regular form available in the market. The statistical methods utilized are relatively straightforward, and there is substantial overlap in the means and standard deviations of results between the kombucha and placebo groups. Relying on subjective patient impressions as study variables limits the trial's robustness. In summary, the authors recommend refining the trial's methodology and analysis to enhance its robustness and make replicating it more challenging.
Regarding the clinical assay from the USA of Mendelson, C., Sparkes, S., et al. (2023) [24]. This clinical study they aimed to explore the potential antihyperglycemic effects of Kombucha in adults with type II diabetes mellitus (T2D). The study involved 12 participants aged 18 or older, following a prospective double-blinded crossover design. Participants were instructed to consume either Kombucha or a placebo (240 ml/day) with dinner for four weeks. The results showed that Kombucha reduced average fasting blood glucose levels compared to the baseline (164 mg versus 116 mg/dl, p=0.035). Only seven participants who completed the entire study were included in the analysis of fasting blood glucose. In summary, the limited number of participants raises concerns about the robustness of the statistical calculations; thus, this study is considered a pilot trial. Moreover, Kombucha might emerge as a viable alternative for people with diabetes, contending with the challenge of adhering to a routine of consuming only water. However, additional research, such as human clinical trials, is required to confirm these findings.
Finally, concerning Atkinson, FS., Brand-Miller, J., et al. (2023). [25]. The study aimed to compare the Glycemic and insulin responses of a high-carbohydrate meal paired with Kombucha, soda water, or diet soft drink. Significant differences were found in a randomized trial with 11 healthy adults from Australia. Kombucha showed a lower change in plasma glucose from baseline to peak compared to soda water (p=0.003) and diet lemonade (p=0.008). The mean glycemic index (G.I.) values for Kombucha were significantly lower than soda water (p=0.041) and diet drinks (p=0.050). However, caution is advised due to the small sample size and the lack of clinical context when interpreting these findings for the potential long-term impact of Kombucha on individuals with carbohydrate-specific diseases.
In summary, regarding the clinical trials on the benefits of consuming Kombucha at the intestinal microbiota level, Hanna Bergtrom [22] from Sweden (2018) indicates no variation in microbial flora in healthy individuals. The trial of Vladimir Pilipenko [23] from Russia (2022) suggests specific improvements in IBS patients, so more research is needed in this field of study of the microbiota related to the benefits of consuming Kombucha. Concerning the two pilot clinical trials carried out in T2D and healthy individuals concerning Mendelson-Sparkes's research [24] and Atkinson Brand-Miller [25], the results are encouraging, reflecting the antidiabetogenic power demonstrated in animals, but with this small number of participants, it makes extrapolation of results difficult, so more clinical trials are necessary around diabetes.
Conclusion
Our analysis of existing literature reveals a dearth of affirmative outcomes in human studies, underscoring the need for more scientifically sound articles. Some clinical trial results on microbiota are contradictory and debatable, necessitating further investigation, particularly to fathom the beneficial impacts of consuming kombucha. Although recent preliminary positive results in diabetes research are encouraging, it is important to exercise caution due to limited documentation. It would be beneficial to conduct a trial assay backed by a government agency or a reputable organization to explore the potential advantages of consuming this functional beverage without any industry influence or profit motives.
List of abbreviations
SCOBY: Symbiotic culture consortium of bacteria and yeast, NIH: National Institute of Health, NLL: National Library of Medicine, BIREME: Latin America and Caribbean Center for Information on Health Science, LILACS: Latin American and Caribbean Literature on Health Science, MeSH: Medical Subject Healthings, Kappa: Cohen's Kappa agreement coefficient, PCR: Polymerase Chain Reaction, ANOVA: Analysis of Variance, IBS: Irritable Bowel Syndrome, Prisma: Preferred Reporting Items for Systematic Reviews and Meta-Analyses, T2D: diabetics type 2, G.I.: Glycemic index, II: Insulin index,
Declaration of Competing Interest
The authors certify that they have no known conflict of personal relationships or financial interests that may have influenced the work presented in this paper.
Authors’ contributions
Carlos Aulesa, an experienced researcher with a Ph.D. (C.A), and Carmen Gongora, a researcher in training (C.G), contributed valuable contributions to this manuscript. Each author drafted specific sections, and both actively participated in the revision process for the entire document.
Acknowledgments
We want to thank the journalist Alba Aulesa for her collaboration in correcting the original.
Transparence Declaration
The authors assert their commitment to upholding the highest standards of integrity in presenting the study's findings. The adherence to CONSORT/STROBE/PRISMA guidelines ensures clarity and completeness in reporting the qualitative research methodology.
Furthermore, the lead author emphasizes that deviations from the originally planned study have been carefully documented and elucidated. Transparency in reporting is prioritized to provide readers with a comprehensive understanding of the research process.
References
Jayabalan, R., Malbasa, R., Loncar, E., Vitas, J., & Sathishkumar, M. (2014). Review on Kombucha Tea, Microbiology, Composition, Fermentation, Beneficial Effects, Toxicity, and Tea Fungus. Comprehensive Review in Food Science and Food Safety, vol 13(4),538-550. https://doi.org/10.1111/1541 4337.12073View
Abaci, N., Senol Deniz, F. S., and Orhan, I. E., (2022). Kombucha- an ancient fermented beverage with desired bioactivities: a narrowed review. Food Chem X. 14:100302. https://doi.org/10.1016/j.fochx.2022.100302View
Batista, P., Rodriguez Penas, M.,Pintado, M., & Oliveira Silva, P. (2022). Kombucha: Perceptions and Future Prospects. Foods,11(13):1977. https://doi.org/10.3390/foods11131977View
Kim, J., Adhikari,K. ( 2020). Current Trends in Kombucha: Marketing Perspectives and the Need for Improved Sensory Research. Beverages 6(1), 15. https://doi.org/10.3390/ beverages6010015View
Cesar da Silva, J., Meireles-Mafaldo, M., Lima Brito, I., & Tribuzy de Magalh, A., (2022). Kombucha: Formulation, chemical composition, and therapeutic potentialities. Current Research in Food Science, 5:360-5. https://doi.org/10.1016/j. crfs.2022.01.023View
Marco, M., Sanders, M., Ganzle, M., Arrieta, M., Cotter, P., Vuyst, L., Hill, C., & Holzapfel, W., et al. (2021). The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented food. Nature Reviews Gastroenterology Hepatology, Vol 18, March 196-208. https://doi.org/10.1038/s41575-020-00390-5 View
Mojtaba Mousavi, S., Alireza Hashemi, S., Zarei, M., Gholami, A., Wey Lai, C., Chiang Hung, W, Omidifar, N., Baharani, S, Mazraedoost, S. (2020). Recent Progress in Chemical Composition, Production, and Pharmaceutical Effects of Kombucha Beverage: Evid Based Complement Alternative Medicine. Vol 2020, Article ID 4397543;1-14. https://doi. org/10.1155/2020/4397543View
Kurnia Permatasari, H., Nurkolis, F., Satria Augusta, P., Silvia Wewengkang, D., Chairiyah Batubara, S., & Ben Gunawan, W., et al.(2021). Kombucha tea from sea grapes (Caulerpa racemosa) potential as a functional anti-aging food: in vitro and in vivo study. Heliyon,Vol 7, Issue 9, E07944,Sept. https://doi. org/10.1016%2Fj.heliyon.2021.e07944View
Kaewkod, T., Sangboonruang, S., Khacha Ananda,S., Charoenrak, S., Bovonsombut, S., & Tragoolpua, Y., (2022). Combinations of traditional kombucha tea with medicinal plant extracts for enhancement of beneficial substances and activation of apoptosis signaling pathways in colorectal cancer cells. Food Sci and Technol. 42,e107521:1-15. https://doi.org/10.1590/ fst.107521View
Ernest, E. (2003). Kombucha: A systematic review of the clinical evidence. Forsch Komplementarmed Klass Naturheilkd / Research in Complementary and Classical Natural Medicine, Apr;10(2):85-87. https://doi.org/10.1159/000071667View
Vina, I., Semjonovs, P., Linde, R., Deninna, I., (2014). Current Evidence on Physiological Activity and Expected Health Effects of Kombucha Fermented Beverage. J Med Food,17 (2): 179 188. https://doi.org/10.1089/jmf.2013.0031View
Martínez Leal, J., Valenzuela Suárez, L., Jayabalan, R., Huerta Oros, J., & Escalante-Aburto, A., et al. (2018). A review of health benefits of kombucha nutritional compounds and metabolites, CyTA Journal of Food 2018, vol 16,1: 390-399, https://doi.org/ 10.1080/19476337.2017.1410499View
Kapp, J. M., & Sumner, W., (2019). Kombucha: a systematic review of the empirical evidence of human health benefit. Annals of Epidemiology, vol 30. Feb: 66-70. https://doi.org/10.1016/j. annepidem.2018.11.001View
Morales, D., (2020). Biological activities of kombucha beverages.: The need for clinical evidence. Trends in Food Science and Technology, vol 105, Novem: 323-333. https://doi. org/10.1016/j.tifs.2020.09.025View
Krieger Vargas, B., Fensterseifer Fabrucio, M., Zachia Ayub, M., (2021). Health effects and probiotic and prebiotic potential of Kombucha: A bibliometric and systematic review. Food Bioscience, vol 44(A):Decem.101332. http://dx.doi. org/10.1016/j.fbio.2021.101332View
Esatbeyoglu, T., Sarikaya Aydin, S., Gultekin Subasi, B., Erskine, E., Gok, R., Ibrahim, S. Y., & Yilmaz, B., et al.(2023). Additional advances related to the health benefits associated with Kombucha Consumption.Critical Review in Food Science and Nutrition, January 20;1-18. https://doi.org/10.1080/104083 98.2022.2163373View
Page, M. J., McKenzie., J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., Shamseer, L., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting Systematic reviews. BMJ;372:n71. https://doi.org/10.1136/bmj. n71View
Cohen, J. A., (1960). Coefficient of agreement for nominal scales. Educ Psychol Meas; vol 20:Issue 1:37-46. http:// doi.org /10.1177/001316446002000104View
Cohen, J., (1968). Weighted kappa: Nominal scale agreement provision for scaled Disagreement or partial credit. Psychological Bulletin, 70(4), 213–220. https://doi.org/10.1037/h0026256View
Byrt, T., Bishop, J., Carlin, J. B., (1993). Bias Prevalence and kappa. J.Clin Epidemiol, vol 46,(5):423-9. https://doi. org/10.1016/0895-4356(93)90018-VView
Byrt, T., (1996). How good is that agreement?. Epidemiology,sep;7(5):561. https://doi.org/10.1097/00001648 199609000-00030View
Bergström, H., Hakansson, A., Rosenstock, N., et al. (2019). The Effect of the Fermented Tea Beverage Kombucha on the Oral and Gut Microflora. A double-blind placebo-controlled. https://lup.lub.lu.se/luur/download?func=downloadFile&recor dOId=8954225&fileOId=8954227View
Pilipenko,V. I., Isakov, V. A., Morozov, S.V., Vlasava, A. V., & Kochetkov, A. A. et al.(2022). New Specialized Kombucha Based Non-alcoholic Pasteurized Beverage Shows Favorable Profile of Short-Term Safety and Tolerability in Patients With Constipation. Current Developments in Nutrition ,vol 6(1):529 6(supplement 1):529. https://clinicaltrials.gov/show/ NCT05164861.View
Mendelson, C., Sparkes, S., Merenstein, D. J., C Christensen, C., Sharma,V., Desale, S., Auchtung, J. M.., Kok et al.(2023). Kombucha tea as an antihyperglycemic agent in humans with Diabetes: a randomized controlled pilot investigation. Front. Nutr.vol10:Aug1190248. https://doi.org/10.3389/ fnut.2023.1190248View
Atkinson, F. S., Cohen, M., Lau, K., & Brand-Miller, J. C., (2023). Glycemic and insulin index after A standard carbohydrate meal consumed with live Kombucha: A randomized, placebo controlled, crossover trial. Front Nutr .vol 10, Feb.1036717. https://doi.org/10.3389/fnut.2023.1036717View